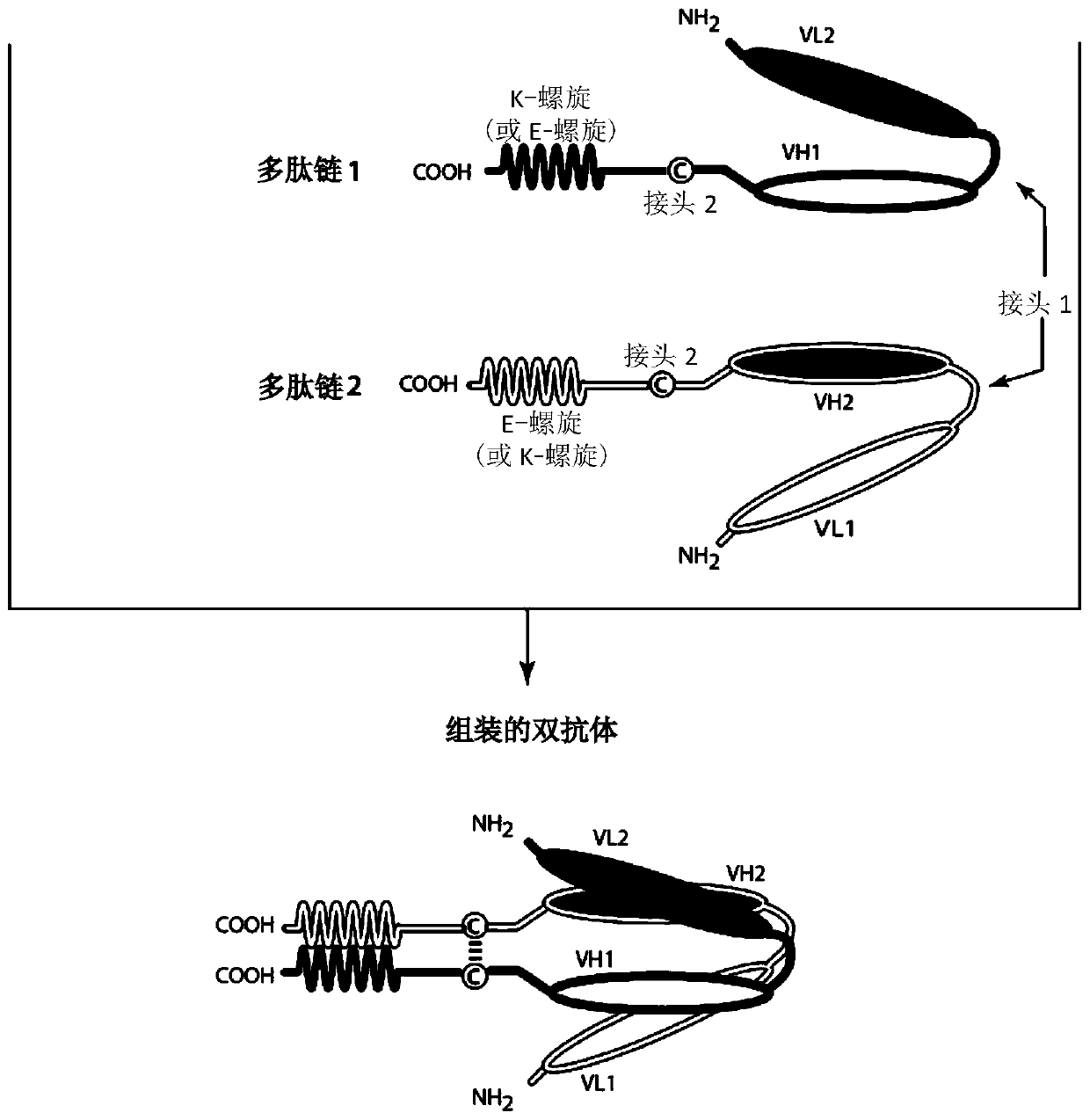
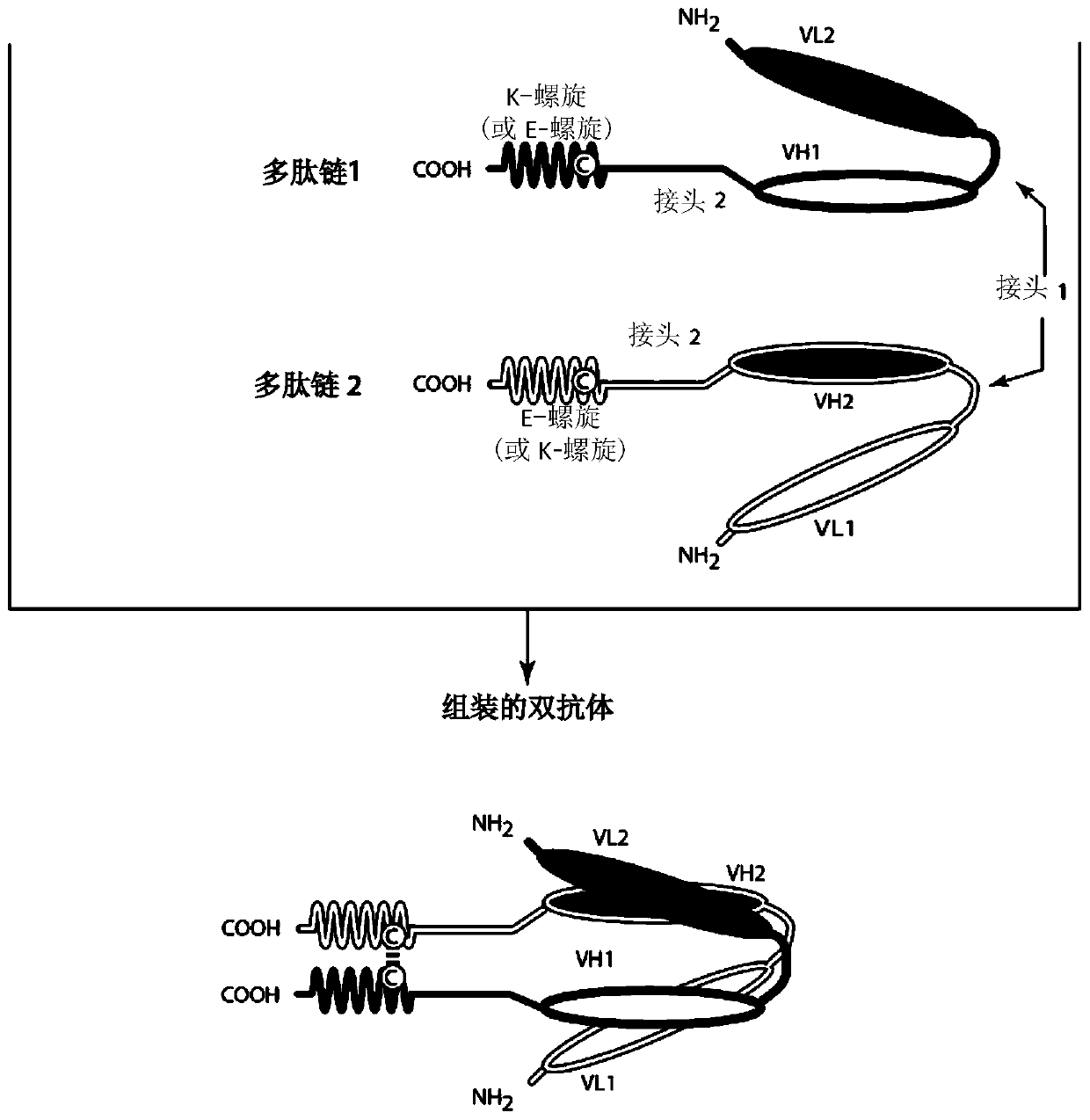
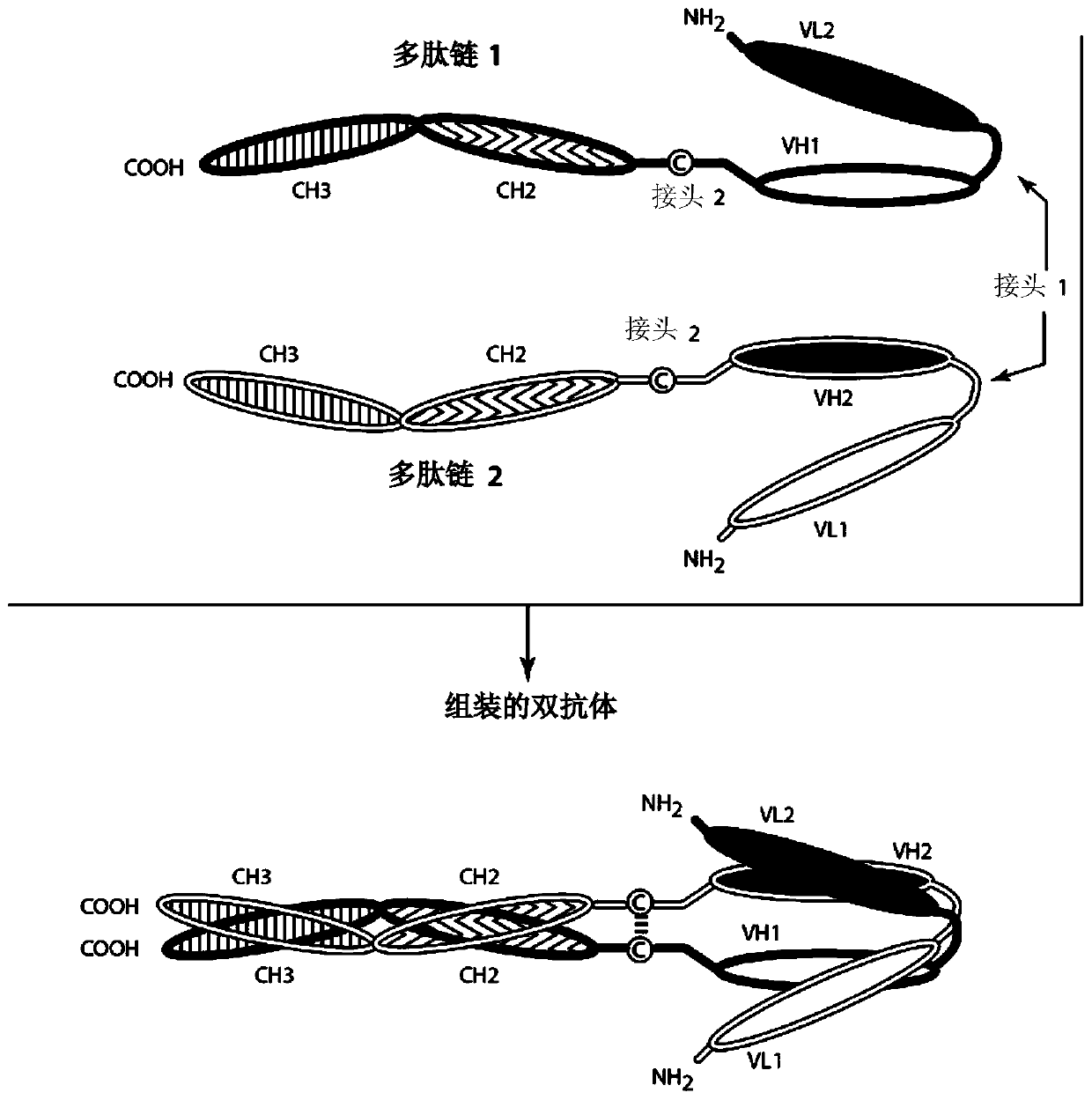
Bispecific binding molecules that are capable of binding cd137 and tumor antigens, and uses thereof
A technology that binds molecules, tumor antigens, and is used in the treatment of cancer and other diseases and conditions, and can solve problems such as obstacles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1229] Humanization of HER2 MAB-1
[1230] PCT Publication WO 2001 / 036005 discloses an anti-HER2 / neu antibody, designated "8H11" therein, however, the sequences of the VL and VH domains provided therein are inaccurate. The 8H11 antibody binds to a HER2 / neu epitope that is different from that bound by trastuzumab, margotuximab, and pertuzumab.
[1231] Deriving the correct sequences of the VH and VL domains of the anti-HER2 / neu antibody 8H11 (thus providing HER2-MAB-1 VH (SEQ ID NO:60) and HER2-MAB-1 VL (SEQ ID NO:61)), And multiple rounds of humanization were performed to obtain the above-mentioned humanized VH domain "hHER2 MAB-1 VH1" (SEQ ID NO:64) and humanized VL domain "hHER2 MAB-1VL1" (SEQ ID NO:67 ). During this effort, several potential sequence liabilities were identified in the hHER2 MAB-1 VH1 and hHER2 MAB-1 VL1 domains, and substitutions were made that removed these liabilities while maintaining binding affinity. especially:
[1232] (1) The aspartic acid isome...
Embodiment 2
[1241] Isolation and Characterization of Mouse Anti-CD137 mAb
[1242]A panel of murine monoclonal antibodies specific for human CD137 was screened for antibodies exhibiting high affinity binding. Three antibodies were selected for further study. For this assessment, purified pan T-cells (see above) were stimulated for 72 hours with anti-CD3 / CD28 beads (cell:bead = 1:1) in the presence of IL-2 (100 U / mL). 100 μL of activated T cells (1.0x10 6 cells / mL) and 100 μL of serially diluted (5-fold or 10-fold dilution) test article (antibody or bispecific diabody) were added to each well of a microtiter assay plate, mixed and incubated at room temperature for 45 minutes. Cells were washed with FACS Buffer and then secondary antibody (anti-human-Fc region-APC) was added to each well; mixed and incubated at room temperature for 30 minutes. Cells were then washed with FACS Buffer and then T cell surface marker antibodies (V510-labeled anti-CD4, and FITC-labeled anti-CD8) were added to...
Embodiment 3
[1256] Humanization of mouse anti-CD137 mAb
[1257] Antibodies CD137 MAB-3 and CD137 MAB-4 were selected for humanization. Four rounds of humanization were performed on CD137 MAB-3, which resulted in one humanized VH domain, designated "hCD137 MAB-3 VH1" (SEQ ID NO:76), and three humanized VL domains, designated "hCD137 MAB-3 VL1" (SEQ ID NO:87), "hCD137 MAB-3 VL2" (SEQ ID NO:88), and "hCD137 MAB-3 VL3" (SEQ ID NO:89).
[1258] Three rounds of humanization were performed on CD137 MAB-4, which resulted in one humanized VH domain, designated "hCD137MAB-4 VH1" (SEQ ID NO:92), and two humanized VL domains, designated " hCD137 MAB-4 VL1" (SEQ ID NO:94) and "hCD137 MAB-4 VL2" (SEQ ID NO:95).
[1259] Humanized heavy and light chain variable domains with a particular anti-CD137 antibody (eg, CD137 MAB-3) can be used in any combination and specific references to humanized chains are made by reference to specific VH / VL domains. A combination, eg, a humanized antibody comprising hCD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com