Ccr2+hematopoietic stem cells mediate t cell activation in adoptive cell therapy
A technology of adoptive cell therapy and hematopoietic stem cells, which is applied in the direction of anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, animal cells, tumor/cancer cells, etc., and can solve problems such as no clinical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1. Bone marrow-derived monocyte populations are highly heterogeneous and distinct subpopulations are defined based on the differential expression of various bone marrow markers.
[0076] The chemokine receptor CCR2 is expressed on monocyte precursor cells and is required for entry into the CNS, often in chemotactic responses to CCL2, which is expressed by KR158B gliomas (Clarkson et al., 2015; Sagar et al. People, 2012; Flores et al., 2015). In this study, a subset of HSCs was observed to express CCR2 (CCR2 + HSCs) migrated to intracranial tumors within 3 hours (data not shown). To isolate CCR2 + HSC, bone marrow was collected from non-tumor bearing mice and lineage depleted using a magnetic bead isolation kit (Miltenyi Biotec, CA). The resulting lineage-negative HSCs were then further enriched by a second magnetic bead depletion using biotinylated anti-CCR2 antibody followed by anti-biotin bead-conjugated antibody.
Embodiment 2
[0077] Example 2. Determining whether HSC-derived cells found in tumors extravasate from intracranial tumors to tumor-draining lymph nodes and subsequently present tumor antigens to peripheral T cells.
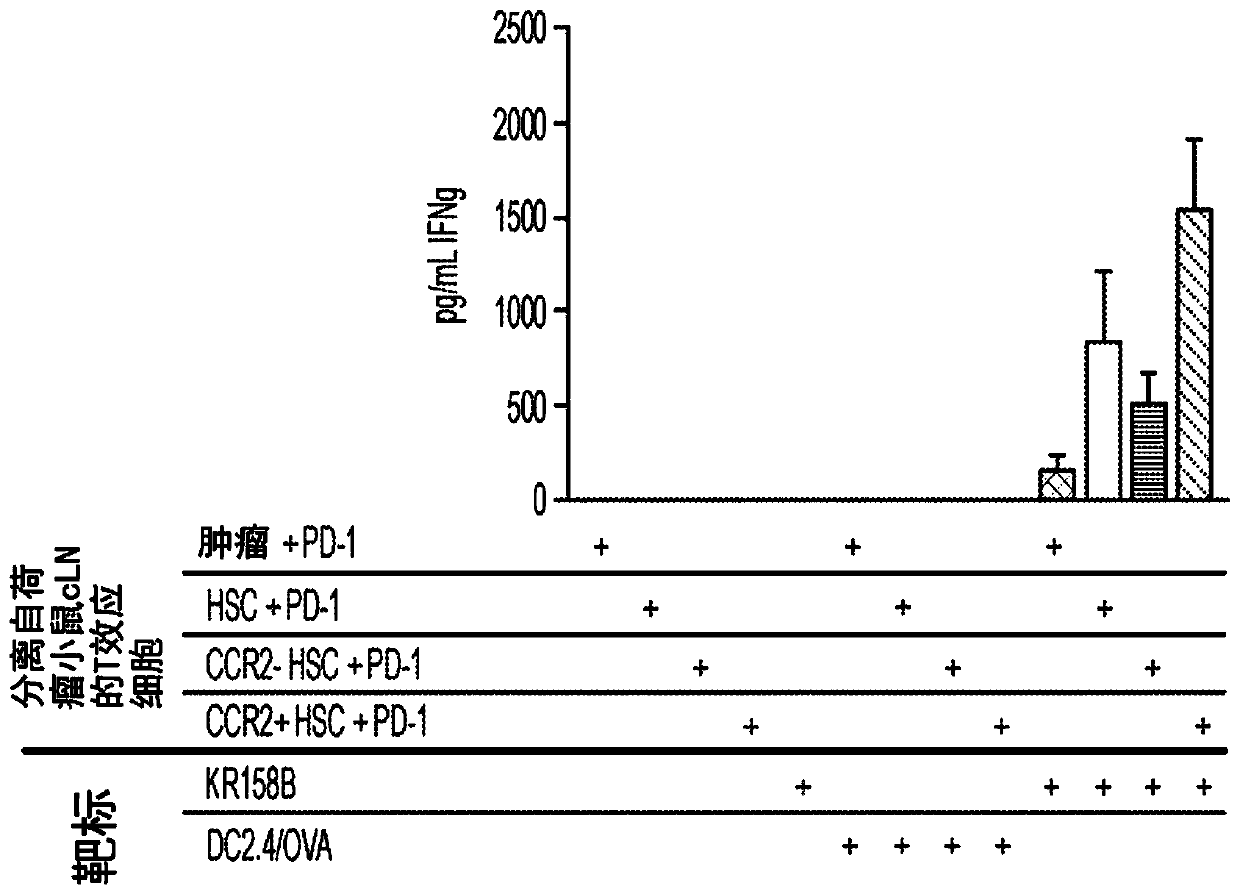
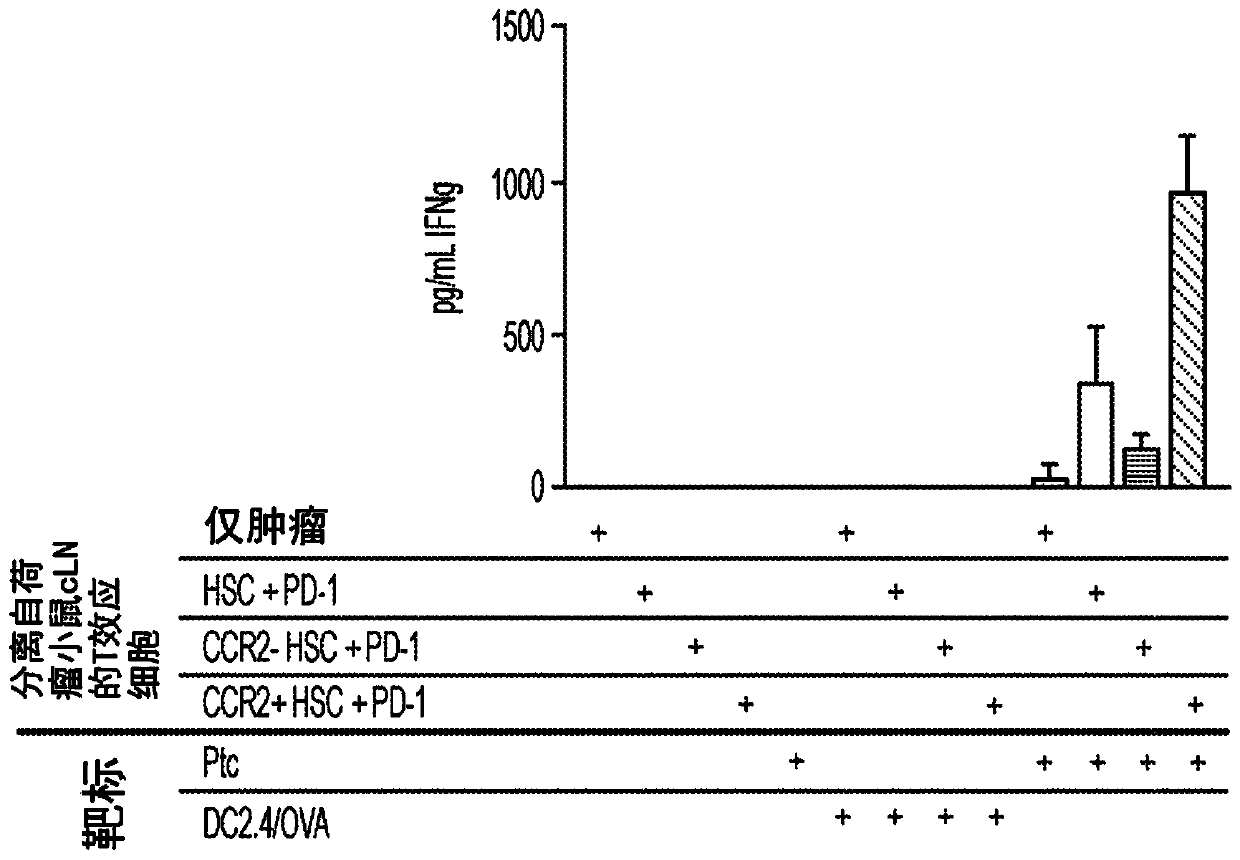
[0078] HSCs were isolated from the bone marrow of non-tumor-bearing mice expressing the DsRed reporter gene on the beta-actin promoter. DsRed + HSCs are further divided into CCR2 + HSC or CCR2 - HSC. DsRed + HSC, DsRed + CCR2-HSC or DsRed + CCR2 + HSCs were injected directly into intracranial KR158B tumors established in vivo. One week later, tumor-draining cervical lymph nodes were collected and analyzed for DsRed + Presence of HSC-derived cells. Accept CCR2 + Group of HSCs had significantly more CCR2 in lymph nodes + HSC-derived cells (p=0.021 vs. unsorted HSC group), demonstrating that CCR2+ HSCs generate cells that preferentially migrate to lymph nodes. To determine the infiltration of CCR2 from the tumor to the lymph nodes + Whether HSC-derived cells have the...
Embodiment 3
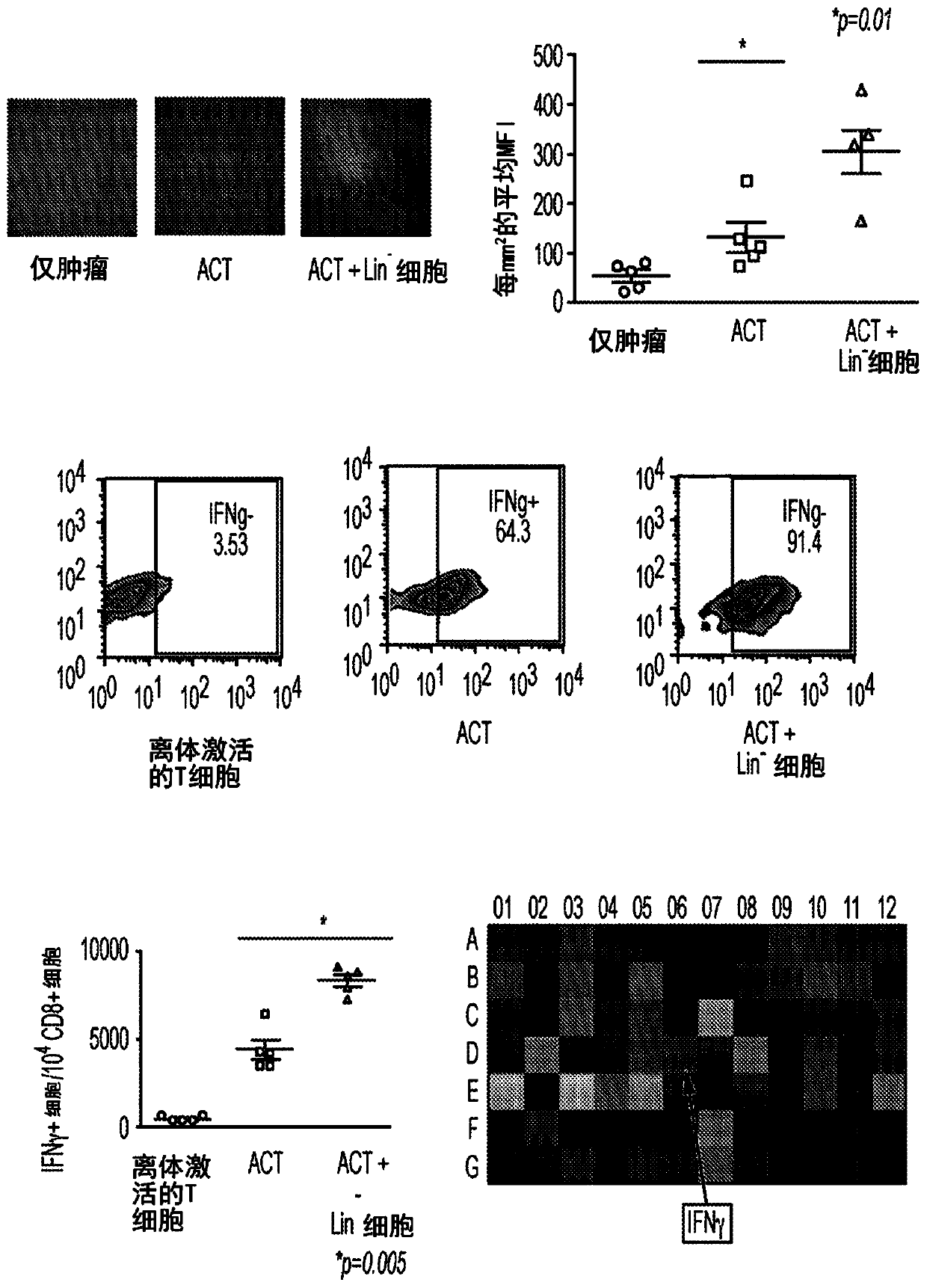
[0079] Example 3. Effect of co-administration of HSC and ACT.
[0080] The inventors previously demonstrated that co-transfer of HSCs with adoptive cell therapy mediates intratumoral migration and engraftment of tumor-specific T cells, as well as inhibits early tumor growth in KR158B gliomas (Flores et al., 2015). Therefore, they evaluated the use of CCR2 + Whether HSCs also enhance the efficacy of adoptive cell therapy (ACT). The ACT platform uses tumor total RNA-pulsed bone marrow-derived dendritic cells to expand tumor-specific T cells (TTRNA-T cells) ex vivo 13 . This study showed that HSC and ACT administration together significantly increased TTRNA-T cell activation and IFNγ secretion in the tumor microenvironment and tumor-draining lymph nodes relative to ACT alone ( figure 2 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com