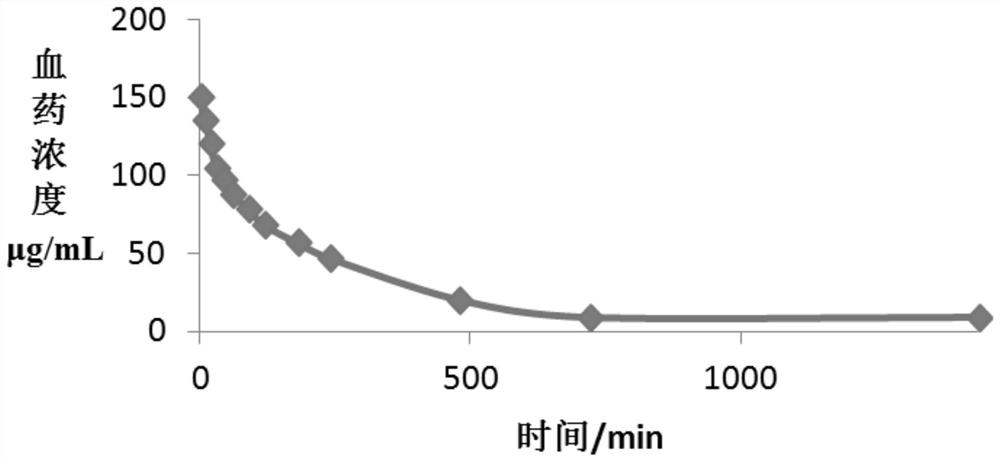
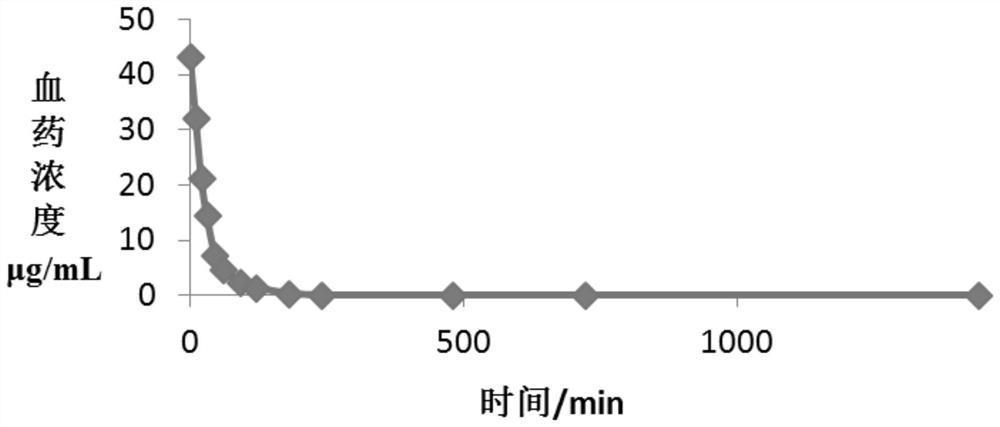
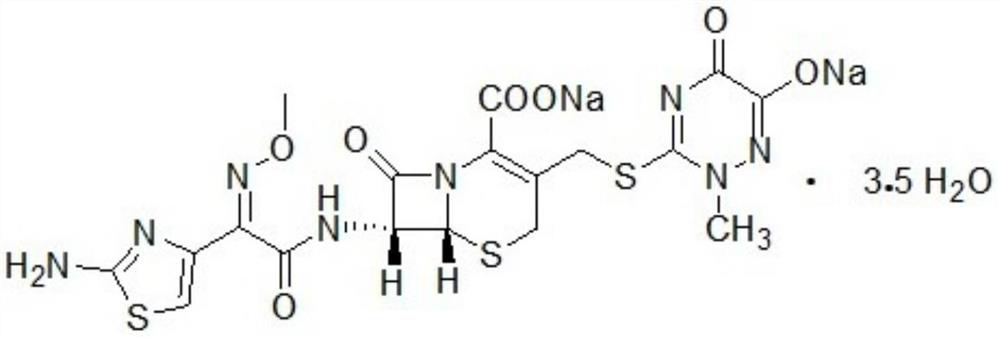
New indication of trisofen ceftriaxone sodium drug preparation in the treatment of infection in immunocompromised patients
A technology of ceftriaxone sodium and compound, applied in the field of drug preparation, can solve the problems of strong acid, strong base, oxidation and temperature instability, poor stability of ceftriaxone sodium preparation, weakened stability of finished preparation, etc., so as to reduce clinical complications. Sensitive risk, beneficial to long-term storage and placement, and the effect of improving safety in use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
[0123] The synthesis of embodiment 1-2 ceftriaxone sodium
[0124] The same as the synthesis process of Example 1-1, the only difference is: in step 1), 14.5gAlCl is added dropwise to the reaction system 3 -BF3-dimethyl carbonate solution [(wBF 3 )=18%] (wherein, AlCl 3 0.64g (0.0048mol), BF 3 2.6g (0.038mol)), that is, aluminum trichloride AlCl 3 -BF 3 AlCl in dimethyl carbonate composite catalyst 3 and BF 3 The weight ratio is 1:8.
[0125] The synthesis of embodiment 1-3 ceftriaxone sodium
[0126] The synthesis process is the same as that of Example 1-1, except that in step 2), the reaction solvent is changed from dichloromethane to acetonitrile, and the crystallization solvent is acetone.
Embodiment 1-4
[0127] The synthesis of embodiment 1-4 ceftriaxone sodium
[0128] The synthesis process is the same as that of Example 1-1, except that in step 3), the reaction solvent is changed from 20% methanol-water for injection to water for injection, and the crystallization solvent is acetone.
Embodiment 2-1
[0129] The synthesis of embodiment 2-1 sulbactam sodium
[0130]Step A), 330mL (3.6mol) of 25% hydrobromic acid was cooled to 0°C, and the pH was adjusted to about 2.5 by adding dilute sulfuric acid dropwise; 120mL water and 108g 6-APA (0.5mol) were weighed to prepare a suspension, Cool to 5°C, add dropwise to the hydrobromic acid solution; weigh 34.5g (0.5mol) of sodium nitrite and dissolve it in 55mL of water, add dropwise to the above system, keep stirring during the dropwise addition, and continue to stir after the dropwise addition is completed The reaction was carried out for 1.5 hours, and the temperature was controlled at 0-5° C. during the reaction. After the reaction is completed, 14 (weight)% hydrogen peroxide (1.5 mol) is added dropwise to the above system, and the reaction is stirred for 1 hour after the dropwise addition, and the temperature is controlled at 0-5° C. during the reaction. After the reaction was completed, 15% sodium bisulfite was slowly added unti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com