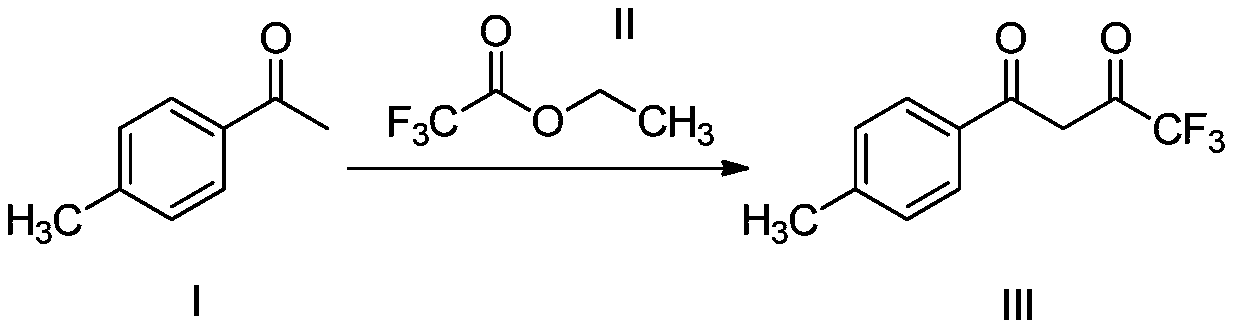
Method for preparing Celecoxib dione intermediate
A technology for celecoxib and intermediates, which is applied in the field of synthesis of small molecular compounds, and can solve the problems of difficulty in obtaining ultra-fine-grained carbonates and large dosages, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 1000ml reaction flask, add 500.0g toluene, 187.9g (1.40mol) p-methylacetophenone, 94.1g sodium methoxide (1.68mol), slowly add 238.8g ethyl trifluoroacetate (1.54mol) dropwise, Keep warm at 20-30°C, continue to react for 5 hours after the dropwise addition, adjust the pH value to 2 with the prepared 5N hydrochloric acid solution, and then wash twice with 500g purified water; the organic phase after washing is slowly cooled to -10°C~- Crystallize at 5°C for 2 hours, filter and dry to obtain 305.5 g of a light yellow solid with a molar yield of 94.8% and a purity of 99.8%.
Embodiment 2
[0022] In a 1000ml reaction flask, add 500.0g toluene, 187.9g (1.40mol) p-methyl acetophenone, 94.1g sodium methoxide (1.68), slowly add 238.8g ethyl trifluoroacetate (1.68mol) dropwise, keep warm 20~30℃, continue to react for 5h after the dropwise addition, adjust the pH value to 2 with the prepared 5N hydrochloric acid solution, and then wash twice with 500g purified water; the organic phase after washing is slowly cooled to -10℃0~- Crystallize at 5°C for 2 hours, filter and dry to obtain 307.1 g of a light yellow solid with a molar yield of 94.8% and a purity of 99.3%.
[0023] The present embodiment adopts different mol ratios, compares with embodiment 1, and its yield improves slightly, but product purity descends slightly.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com