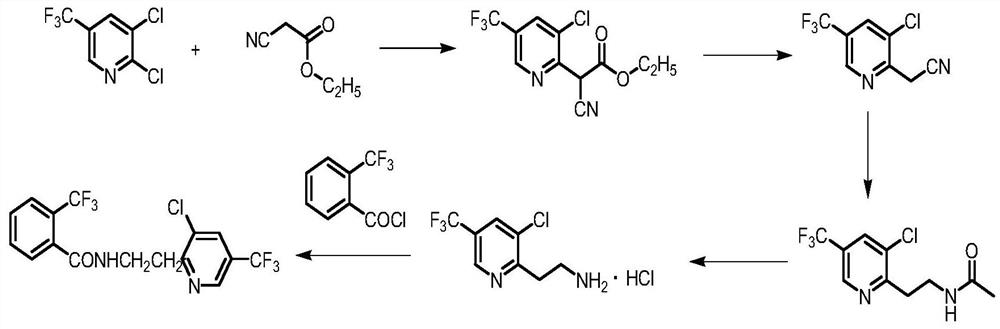
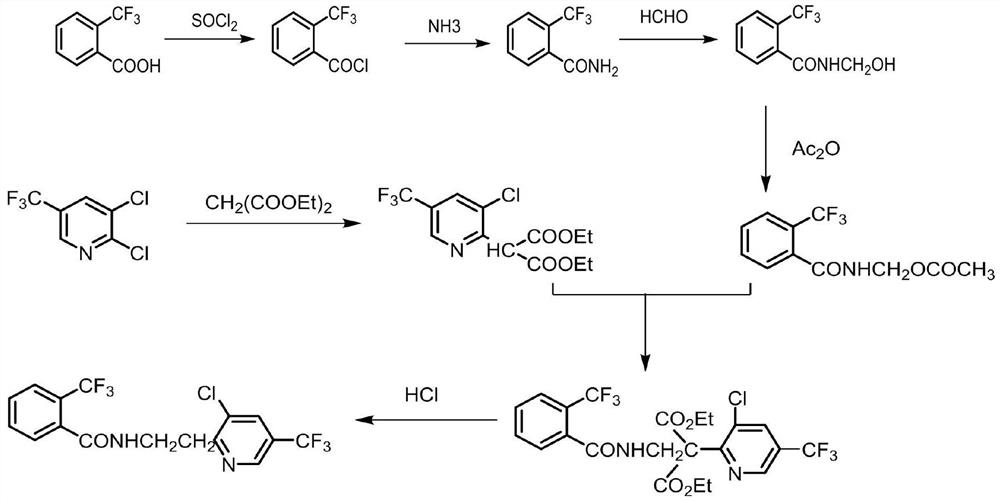
A kind of improved synthetic technique of fluopyram
A fluopyram and synthesis process technology, which is applied in the field of improved synthesis process of fluopyram, can solve problems such as unsatisfactory yield and unfavorable industrialization, and achieve product purity improvement, post-treatment simplification, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Put 2-(3-chloro-5-trifluoromethyl-2-pyridyl)-2-[(2-trifluoromethylbenzoyl)aminomethyl]-1,3- into a 1000mL four-necked bottle Dimethyl malonate 28.5g (0.05mol, 95%, 1.0eq), methanol 150mL, potassium carbonate aqueous solution 125mL (0.25mol, 2M, 5.0eq), heat the system to 60°C and keep it warm for 4h, sample HPLC to detect raw materials The reaction was complete to obtain α-(2-trifluoromethylbenzoyl)aminomethyl-3-chloro-5-trifluoromethyl-2-pyridyl acetate potassium solution;
[0032] Add 200mL of citric acid aqueous solution (0.2mol, 1M, 4.0eq) dropwise to the above system to adjust the pH of the system to 4-5, then keep it warm at 60°C for 4h, and the secondary deacidification reaction controlled by HPLC is complete. The reaction solution was cooled to room temperature 20-25° C., filtered and washed with water to obtain 19.6 g of fluopyram with a content of 99.2% and a yield of 95.0%. The crude product of fluopyram was washed with n-heptane under reflux to obtain a ref...
Embodiment 2
[0034] Put 2-(3-chloro-5-trifluoromethyl-2-pyridyl)-2-[(2-trifluoromethylbenzoyl)aminomethyl]-1,3- into a 1000mL four-necked bottle Dimethyl malonate 28.5g (0.05mol, 95%, 1.0eq), acetonitrile 150mL, sodium hydroxide aqueous solution 100mL (0.4mol, 4M, 8.0eq), the system was heated to 30°C and kept for 3h, and the sample was detected by HPLC The raw materials were reacted completely to obtain α-(2-trifluoromethylbenzoyl)aminomethyl-3-chloro-5-trifluoromethyl-2-pyridyl sodium acetate solution;
[0035]Add 40mL of acetic acid aqueous solution (0.32mol, 8M, 6.4eq) dropwise to the above system to adjust the pH of the system to 4-5, then keep warm at 60°C for 4h, and the secondary deacidification reaction controlled by HPLC is complete. The reaction liquid was cooled to room temperature 20-25° C., filtered and washed with water to obtain 19.1 g of fluopyram with a content of 98.8% and a yield of 93.2%. The crude product of fluopyram was washed with n-heptane under reflux to obtain ...
Embodiment 3
[0037] Put 2-(3-chloro-5-trifluoromethyl-2-pyridyl)-2-[(2-trifluoromethylbenzoyl)aminomethyl]-1,3- into a 1000mL four-necked bottle Dimethyl malonate 28.5g (0.05mol, 95%, 1.0eq), DMF150mL, sodium formate aqueous solution 25mL (0.1mol, 4M, 2.0eq), heat the system to 90°C and keep it for 1h, sample HPLC to detect the complete reaction of raw materials , to obtain α-(2-trifluoromethylbenzoyl)aminomethyl-3-chloro-5-trifluoromethyl-2-pyridyl sodium acetate solution;
[0038] Add 100mL of formic acid aqueous solution (0.2mol, 2M, 1.6eq) dropwise to the above system to adjust the pH of the system to 4-5, then keep it warm at 90°C for 3h, and the secondary deacidification reaction controlled by HPLC is complete. The reaction liquid was cooled to room temperature 20-25° C., filtered and washed with water to obtain 19.8 g of fluopyram with a content of 99.0% and a yield of 96.0%. The crude product of fluopyram was washed with n-heptane under reflux to obtain a refined product with a pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com