Thiazolidone derivative of lovatinib acid and application of thiazolidone derivative
A quinoline formyl thiazolone and compound technology, applied in the field of pharmaceuticals, can solve the problems of unsustainable drug effect, short half-life and the like, and achieve the effects of good safety, low toxicity and excellent anti-cancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
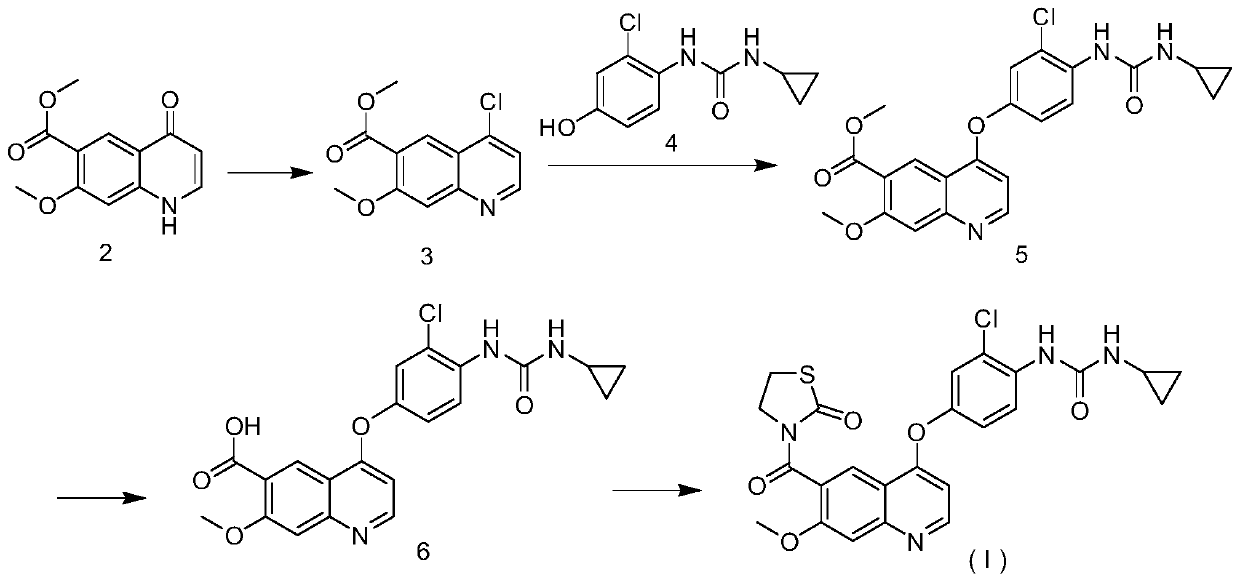
[0018] Example 1: Synthesis of 4-[3-chloro-4-(cyclopropylaminocarbonyl)aminophenoxy]-7-methoxy-6-quinolineformylthiazolone (target compound), see figure 1 ;
[0019] 1.1 Synthesis of methyl 4-chloro-7-methoxyquinoline-6-carboxylate (3)
[0020] 7-Methoxy-4-oxo-1,4-dihydroquinoline-6-carboxylic acid methyl ester (2, synthesis by reference method: Chinese Journal of Medicinal Chemistry, 2015, 285-288) 2.56g was placed in 50mL for drying In an eggplant-shaped flask, 20 mL of thionyl chloride and 3 drops of DMF were added thereto, and stirred under reflux at 125° C. for 3 h. After the reaction was completed, thionyl chloride was spin-dried, and 100 mL of dichloromethane was added thereinto until completely dissolved, then poured into 200 mL of saturated sodium bicarbonate solution, and stirred for 1 h until no bubbles emerged. Extracted, washed once with 100 mL of saturated brine, collected the organic phase, and obtained 0.95 g of a light yellow solid by flash column chromatogr...
Embodiment 2
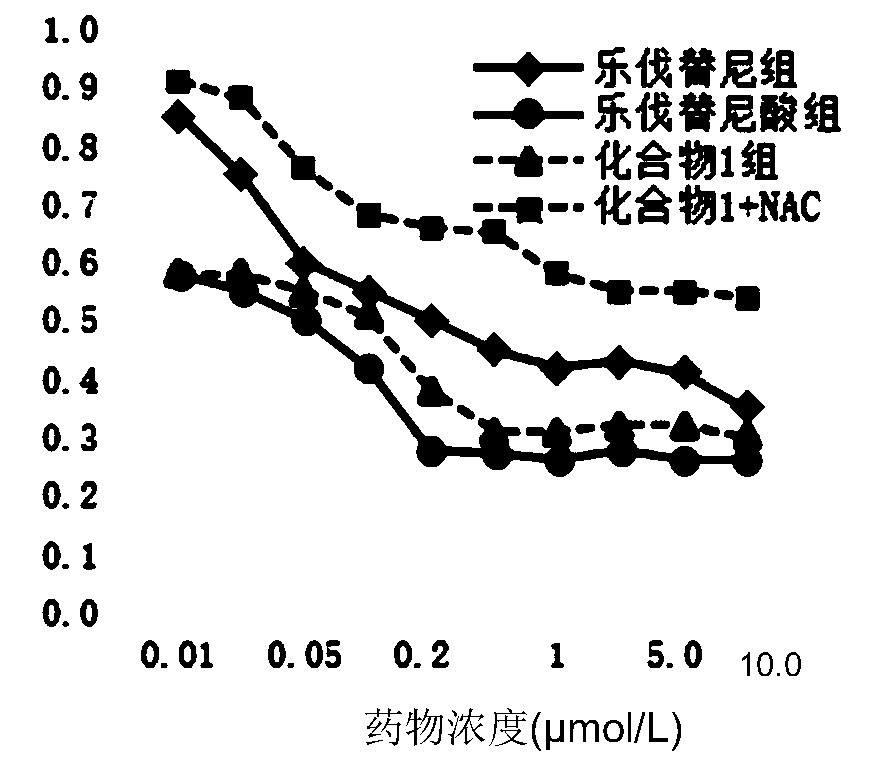
[0028] Example 2: Inhibitory activity screening of target compounds on tyrosine kinases in vitro
[0029] A series of gradient concentrations of the test compound were incubated with a specific concentration of enzyme solution at room temperature for 5 minutes, and then an appropriate amount of enzyme reaction substrate and ATP was added to start the enzyme reaction process. After 30 minutes, an appropriate amount was added to the enzyme reaction system. After incubation for 1 h, measure the enzyme activity at a specific compound concentration on a multifunctional microplate reader, and calculate the inhibitory activity of different concentrations of compounds on the enzyme activity, and then according to the four-parameter equation, for different The inhibitory activity of the enzyme activity at the concentration of the compound was fitted, and the IC50 value was calculated.
[0030] Table 1 Inhibitory activity of target compounds on tyrosine kinases in vitro (IC50, nM)
[0...
Embodiment 3
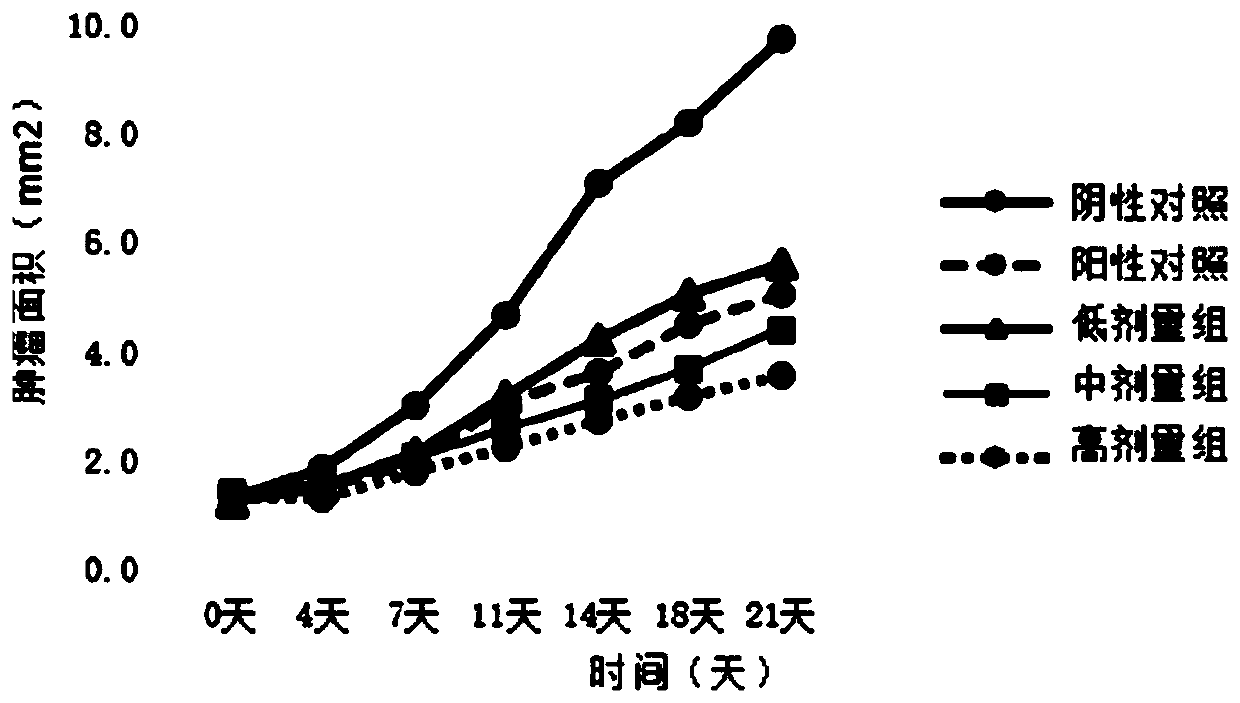
[0033] Embodiment 3: Stability investigation of target compound in normal environment
[0034] Add 1.0 mL of acetonitrile solution of target compound 1 at 100 μmoL / L, add 4.0 mL of PBS buffer solution with pH 7.4, mix well, and measure the peak area of target compound 1 by high performance liquid chromatography; place it at room temperature for 6 and 12 hours, and use high performance liquid The peak area of the target compound 1 was determined by phase chromatography, and compared with the peak area at 0 hours. get the relative value.
[0035] The stability investigation of the target compound of table 2 in the PBS buffer solution of pH 7.4 6 hours and 12 hours
[0036] Compound number 6 hours 13 hours 1 92% 85%
[0037] The above experimental results show that the target compound 1 is relatively stable in PBS buffer solution with pH 7.4 and is not easily hydrolyzed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com