Friedelane type compound as well as preparation method and application thereof
The technology of a compound and a deuterated compound, which is applied in the field of suberane-type compounds and their preparation, can solve the problem that the inhibitory effect cannot meet the demand and the like, and achieves the effects of a green preparation method, improved effect, and strong inhibitory effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
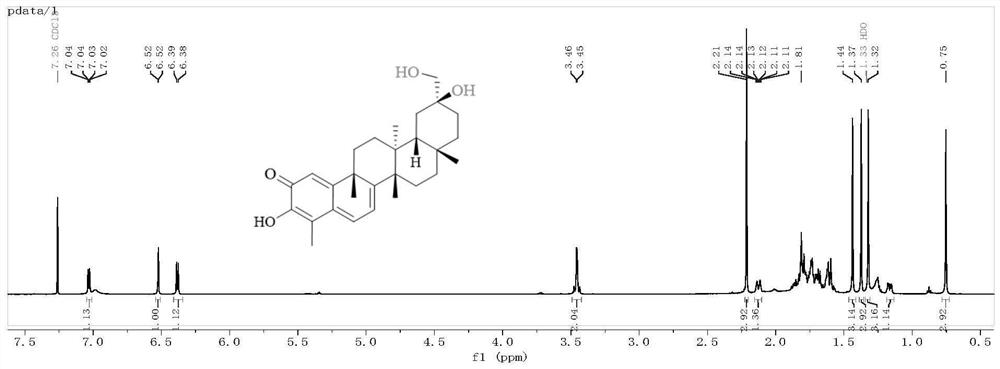
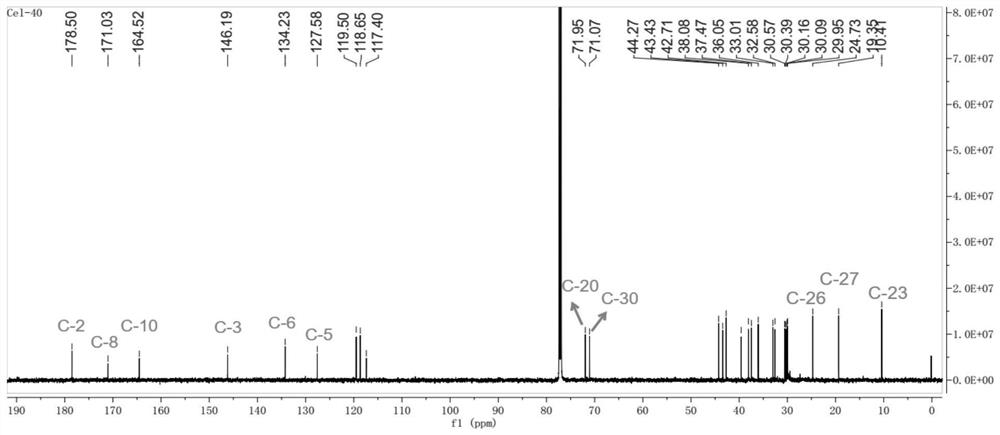
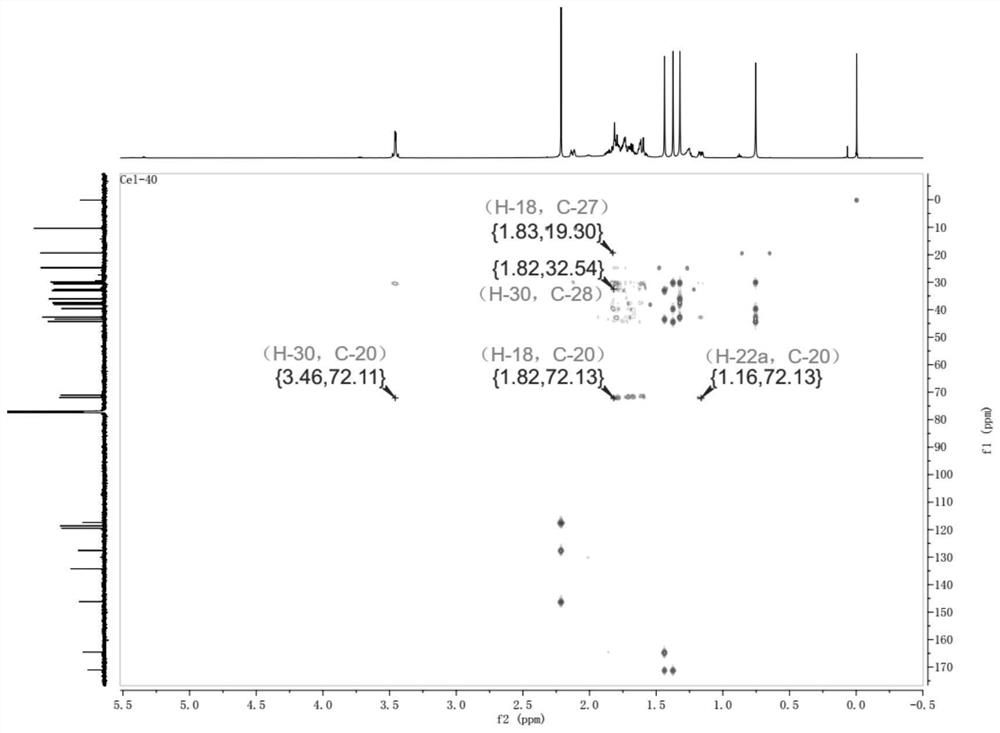
[0038] Embodiment 1, preparation compound CEL-2
[0039]
[0040]Step 1: Take 400mg tripterine, dissolve it in 40ml absolute ethanol, and prepare a 10mg / ml substrate solution. The two-step culture method was used in soybean meal liquid medium (recipe: commercially available soybean meal 5g, glucose 20g, yeast extract 5g, NaCl 5g, K 2 HPO 4 5g, distilled water to 1L) to culture Streptomyces griseus to the logarithmic growth phase (cultivation conditions are 28 ℃, shaker speed 180rpm / min), then add the above substrate solution (control the final concentration of tripterine to 15mg / min). 250mL), continue to cultivate for 4 days and then finish the fermentation, take the fermentation supernatant and extract 3 times with an equal volume of ethyl acetate, recover the organic solvent under reduced pressure, and obtain the fermentation product containing compound CEL-2.
[0041] Step 2: The fermentation product obtained in Step 1 is separated and purified by silica gel column chr...
experiment example 1
[0044] Experimental example 1, the specific inhibitory effect of compound CEL-2 on STAT3
[0045] 1. Experimental method
[0046] In this experiment, Western blot was used to analyze the effect of CEL-2 on STAT3 phosphorylation and its downstream proteins (Survivin, MCL-1) and its upstream proteins (JAK-2, p-JAK-2) in HCT-116 cells function, the specific method is as follows:
[0047] IL-6 stimulation was used to induce inflammation in HCT-116 cells. HCT-116 cells were cultured in RPMI / 1640 medium containing 10% FBS. When the cells grew to the logarithmic growth phase, they were digested with 0.25% EDTA trypsin Cells were collected by centrifugation (1200rpm / min for 4min) and diluted 1:3 for passage.
[0048] (1) Detect the inhibitory effect of tripterine (CEL) and CEL-2 on HCT-116 cells by CCK-8 method, and calculate the IC of CEL and CEL-2 on HCT-116 cells 50 , after routine digestion and centrifugation of HCT-116 cells, adjust the concentration of the cell suspension to ...
experiment example 2
[0058] Experimental example 2, the binding dissociation constant of test compound CEL-2 and STAT3
[0059] 1. Experimental method
[0060] In this experiment example, the surface plasmon resonance technology is used to analyze the interaction mode between CEL-2 and STAT3. The specific method is as follows:
[0061] (1) According to the ligand pre-enrichment, select the best concentration of human STAT3 recombinant protein and the most suitable pH condition, dissolve the STAT3 protein in buffers with different pH, and when it flows through the surface of the chip, it can be electrostatically adsorbed. Bind to the carboxyl group on the surface of the CM5 chip, mix 500μg / mL STAT3 protein with different pH sodium acetate buffer (10mM, pH4.5, 5.0, 5.5) to make the final protein concentration 10μg / mL, if there are bubbles , can be removed by centrifugation. The protein solution prepared with different pH sodium acetate buffer was injected onto the CM5 sensor chip, and its binding ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com