Gene therapy for patients with fanconi anemia
A technique for recombining genes and subjects, applied in blood diseases, chemical instruments and methods, genetically modified cells, etc., can solve problems such as no alternative therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
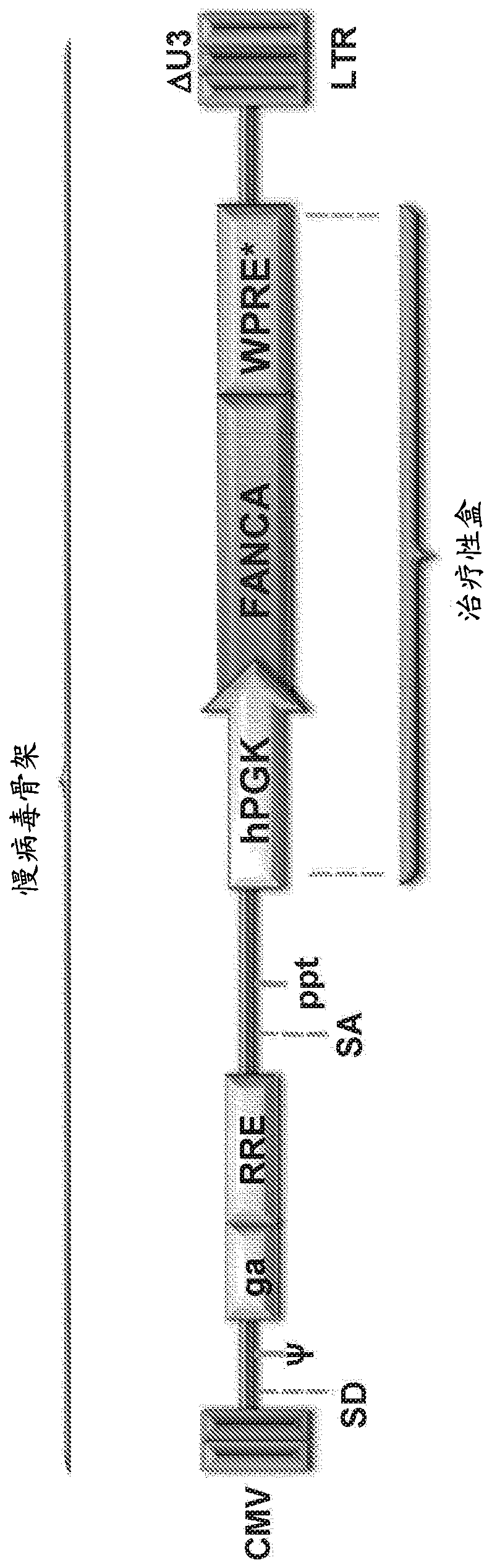
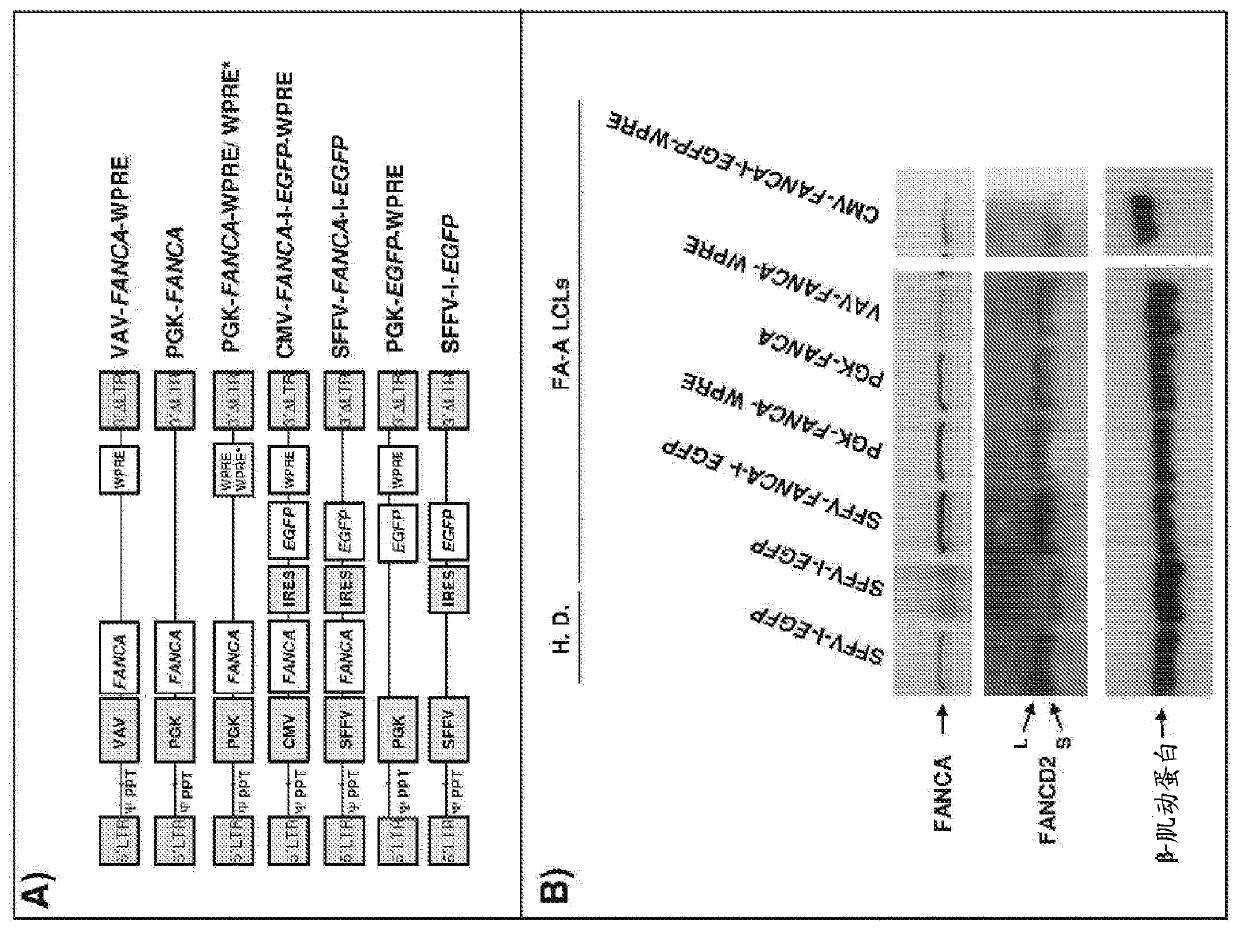
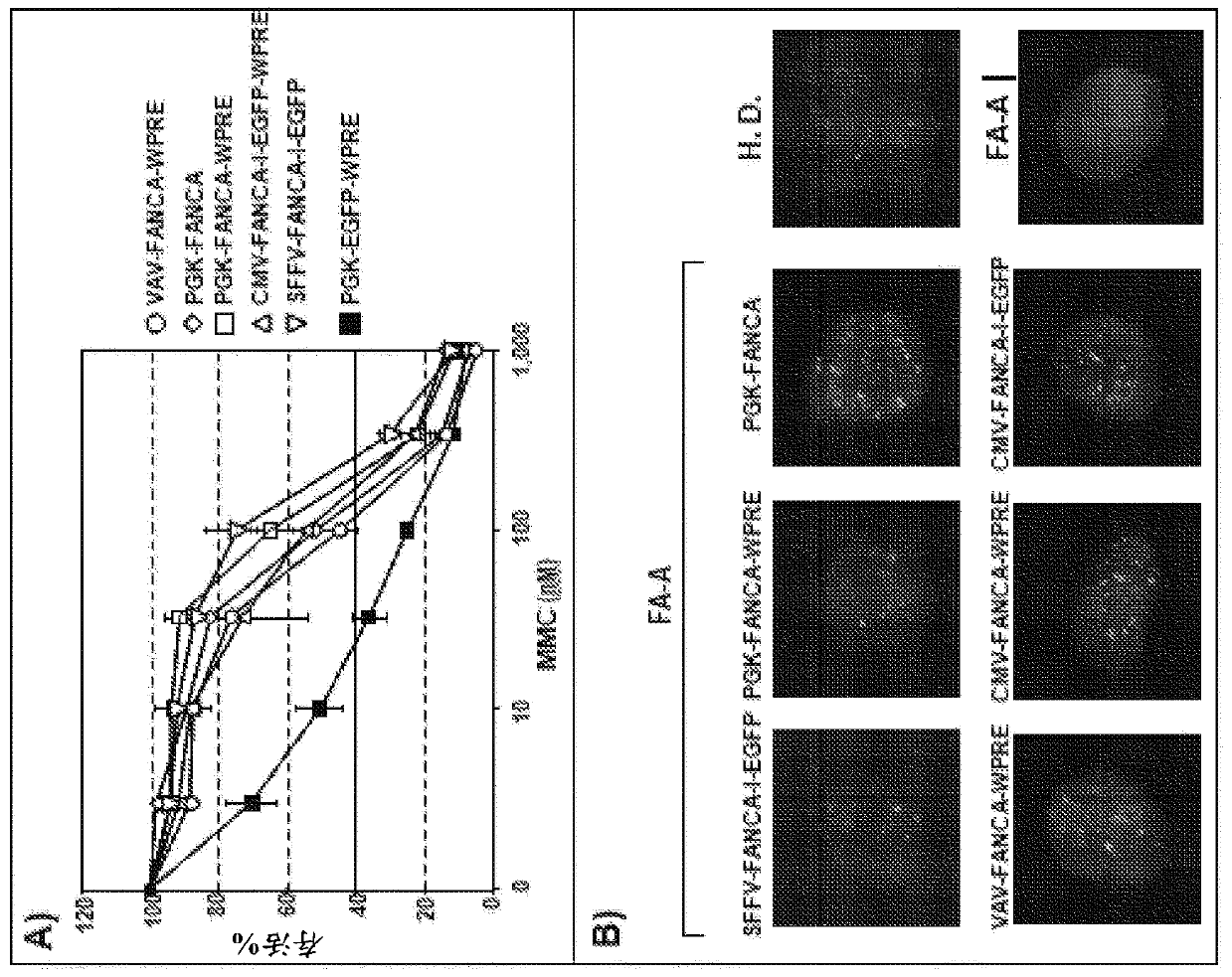
[0286] FANCA lentiviral vector.
[0287] Due to the safer integration pattern of LV compared to RV (Gonzalez-Murillo et al., 2008; Modlich et al., 2009; Montini et al., 2006; Mitchell RS, Beitzel BF, Schroder AR et al. Plos Biol. 2004;2:E234; Montini E et al. J Clin Invest.2009; 119:964-975; Schroder AR et al. Cell.2002; 110:521-529), the purpose of which is to develop LV as a therapeutic vector for correcting FA cell phenotype (Gonzalez-Murillo et al., 2009 ). In addition, since recent studies have shown that LVs with highly efficient internal promoters can also transactivate adjacent genes (Modlich et al., 2009), LVs are considered to be used in the clinic as a link between therapeutic effect and risk of transactivating adjacent genes. compromise between. Therefore, the aim was to define threshold levels of FANCA expression that could be therapeutic to limit the risk of gene transactivation by enhancers / promoters driving therapeutic gene expression. The efficacy of FANC...
Embodiment 2
[0297] Embodiment 2 mouse model research
[0298] To assess the repopulating properties of BM samples from FA patients, whether genetically corrected or not, several groups have transplanted BM cells from FA patients into immunodeficient mice. However, no significant engraftment was reported in any case, most likely because of the reduced number of hematopoietic progenitors and HSCs present in the BM of these patients.
[0299] To evaluate the in vivo effect of the drug, a mouse model containing a deletion of FA-A in the Fanca gene was used. In contrast to FA patients, these animals do not develop overt hematological defects. However, their BM progenitors are highly sensitive to MMC (Rio et al., 2002), also in FA patients. Therefore, in order to determine FANCA LV( Figure 4 A) To stably correct the phenotype of HSCs from FA-A mice in vivo, BM cells from FA-A mice were transduced with FANCA LV and then transplanted into irradiated FA-A recipients ( Figure 4 B). To asse...
Embodiment 3
[0303] Example 3. Efficient transduction of fresh hematopoietic progenitor cells from Cong retro FA patients with lentiviral vectors
[0304] Because transduction of FA cryopreserved hematopoietic stem cells (HSCs) was previously shown to be less efficient compared to transduction of fresh grafts (Jacome et al., 2009), efforts to optimize the transduction efficiency of cryopreserved FA BM samples were undertaken . Such as Figure 7 As shown, performing three transduction cycles significantly increased the transduction efficiency of FA CFCs compared to the value obtained after a single transduction cycle (45.7±4.2% versus 13.5±5.1%, respectively). Samples were subjected to standard transduction (which consisted of a single transduction cycle (16h) after 2 hours of static preloading) (white bars; 1xS) or modified transduction (which consisted of 3 transduction cycles with lentiviral vector Periodic (2h+2h+12h) composition) (gray bar; 3xD).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com