A kind of preparation method of 1,12-dodecanediol
A technology of dodecanediol and dodecanedioic acid, applied in the field of medicine and chemical industry, can solve the problems of difficult separation and purification, low added value of market application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
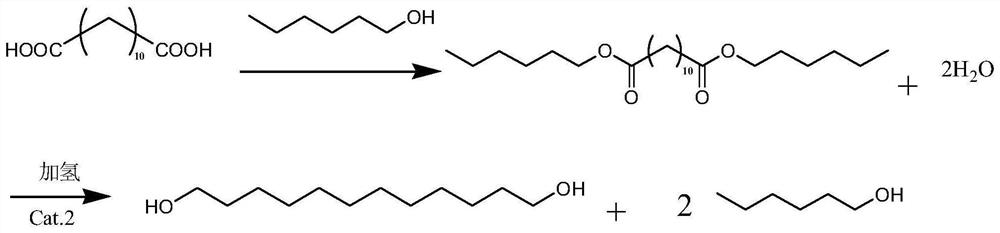
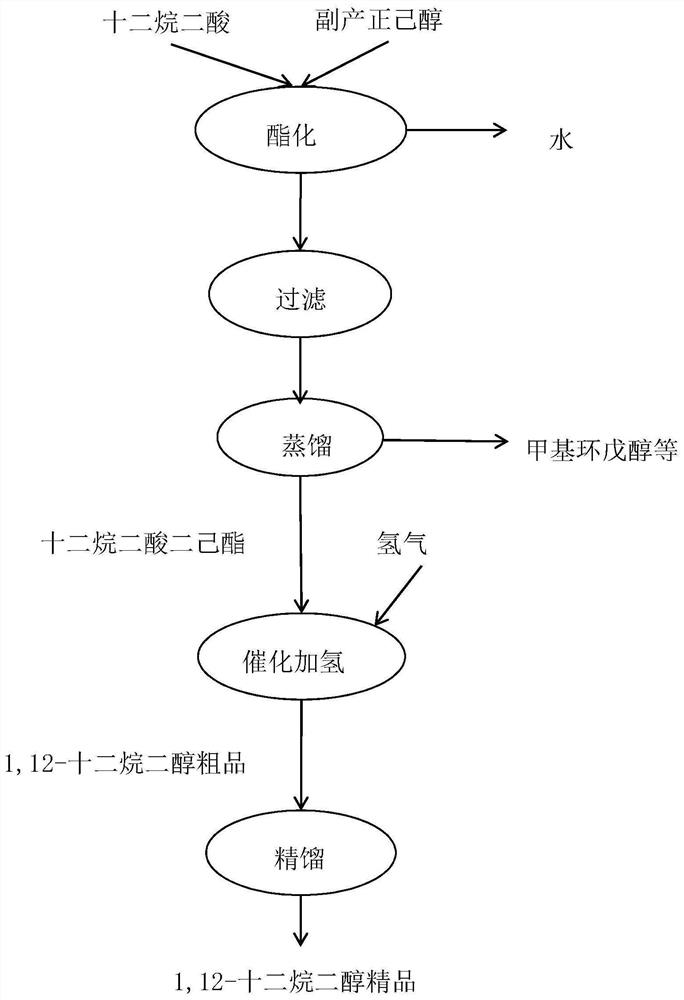
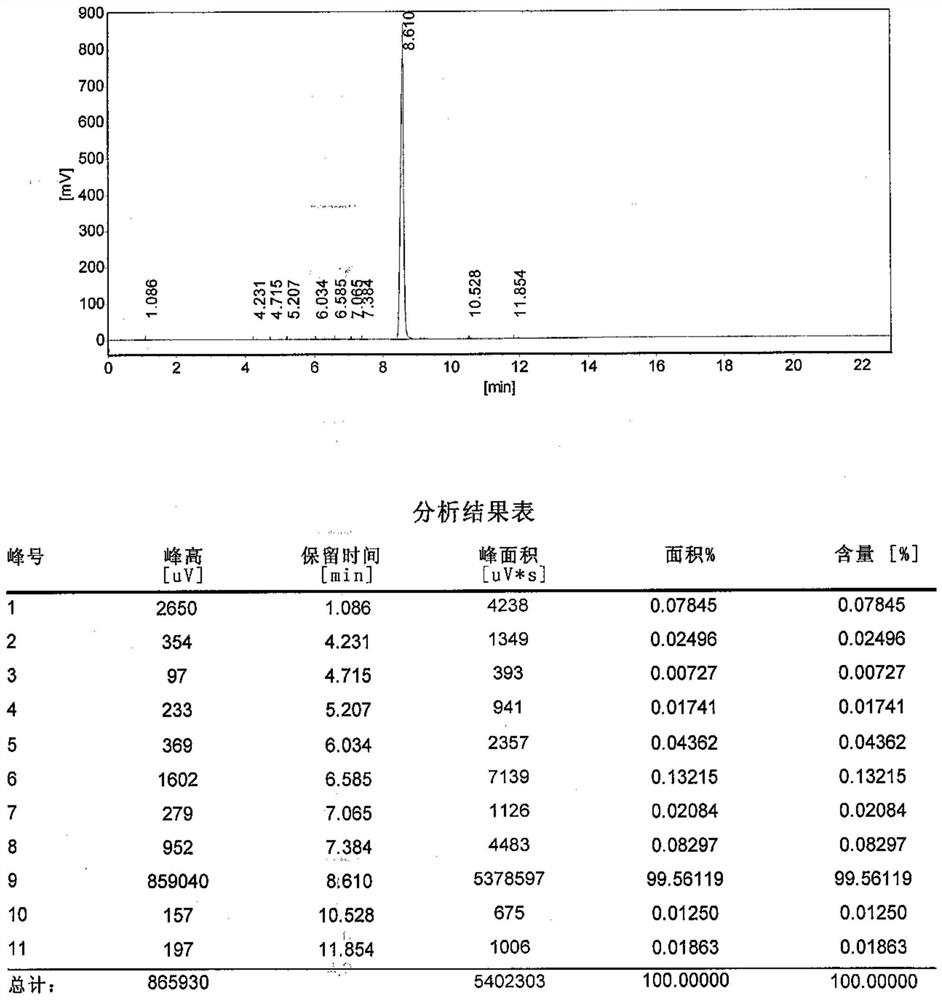
[0039] Example 1: 575 g of dodecanedioic acid and 2875 g of n-hexanol mixture were put into the reaction flask, stirred and heated up. The reaction was refluxed, and the water produced by the reaction was separated. Finally, the temperature was raised to 150°C for reflux reaction, and the reflux water separation reaction lasted for 8 hours. The acid value of the measured sample was 36.1mgKOH / g. After the reaction was completed, the heat preservation and reflux was stopped, and the material was cooled for standby.
Embodiment 2
[0040] Example 2: 575 g of dodecanedioic acid and 2300 g of n-hexanol mixture were put into the reaction flask, stirred and heated up. The reaction was refluxed, and the water produced by the reaction was separated. Finally, the temperature was raised to 170°C for reflux reaction, and the reflux water separation reaction lasted for 9 hours. The acid value of the measured sample was 32.1mgKOH / g. After the reaction was completed, the heat preservation and reflux was stopped, and the material was cooled for standby.
Embodiment 3
[0041] Example 3: 575 g of dodecanedioic acid and 2012.5 g of n-hexanol mixture were put into the reaction flask, stirred and heated up. The reaction was refluxed, and the water produced by the reaction was separated. Finally, the temperature was raised to 185°C, and the reflux and water separation reaction was carried out for a total of 10 hours. The acid value of the measured sample was 30.3 mgKOH / g. After the reaction was completed, the heat preservation and reflux was stopped, and the material was cooled for later use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com