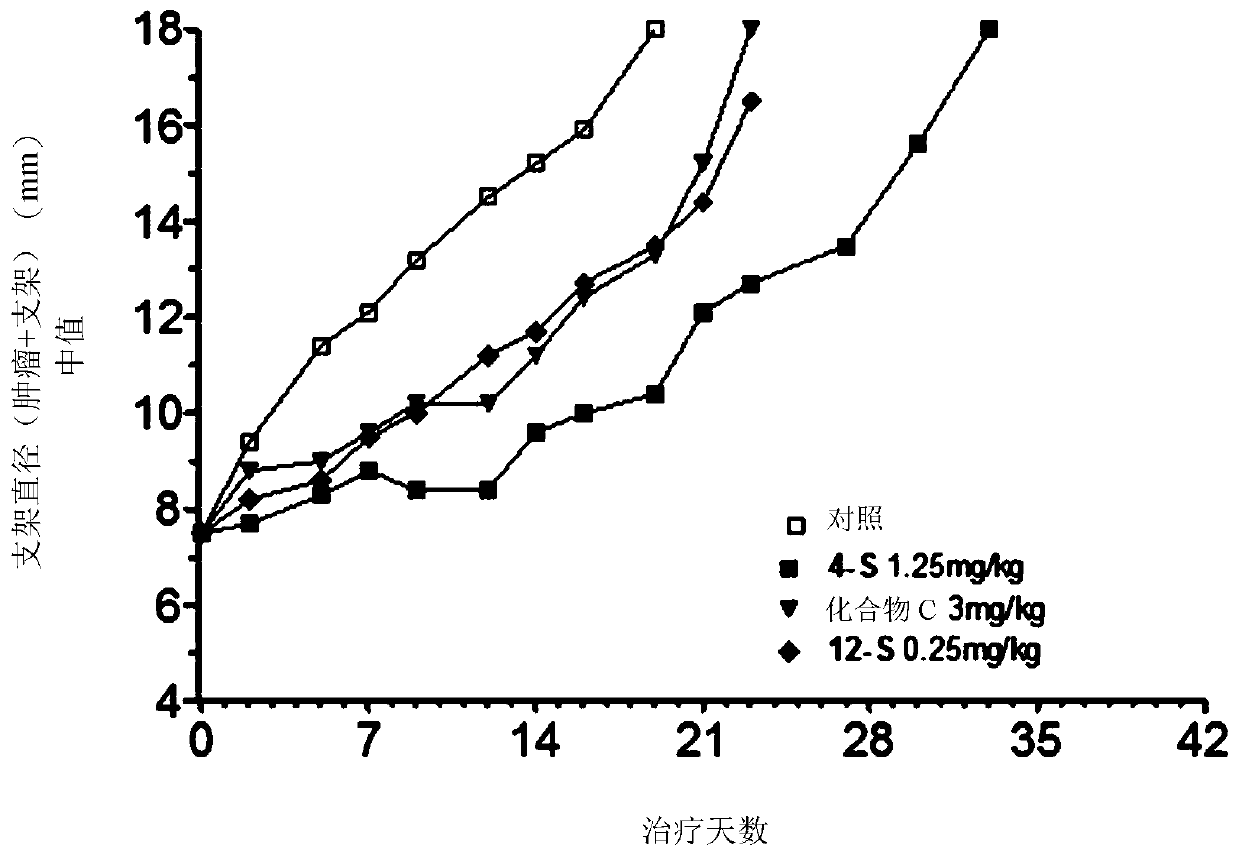
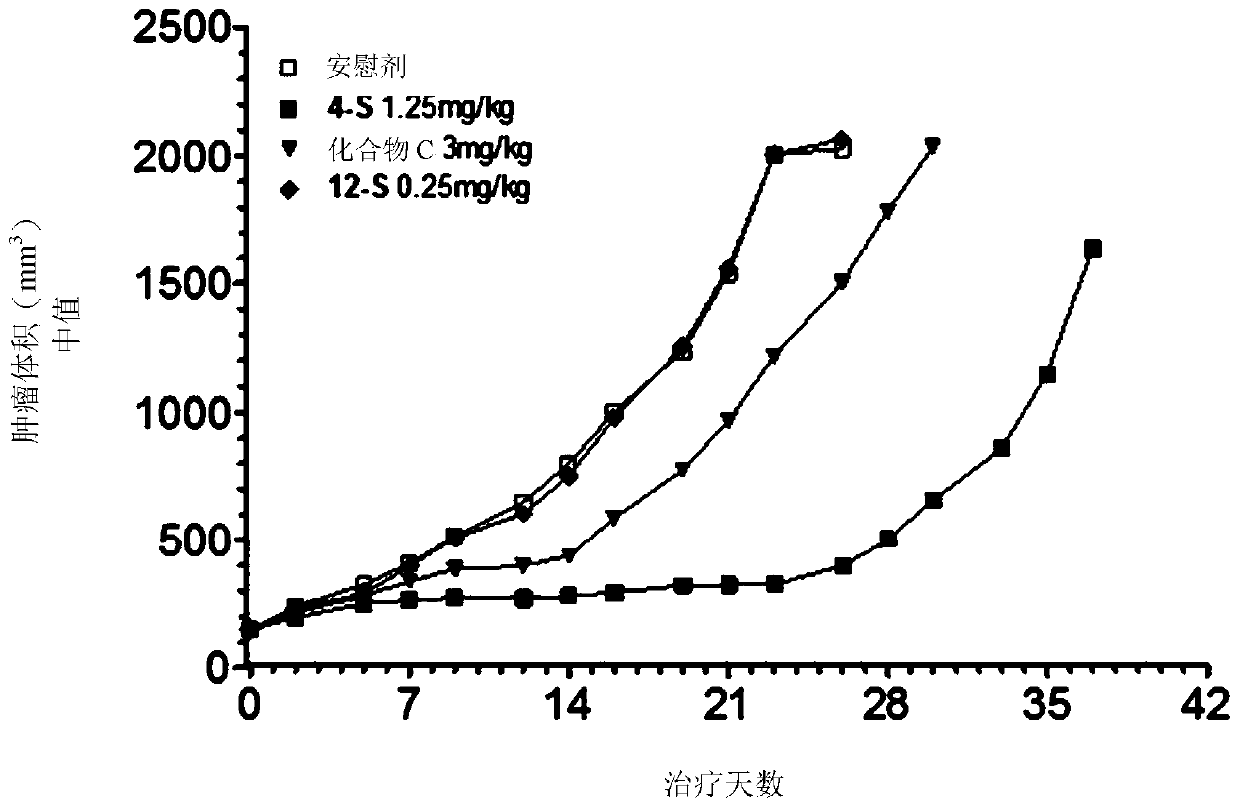
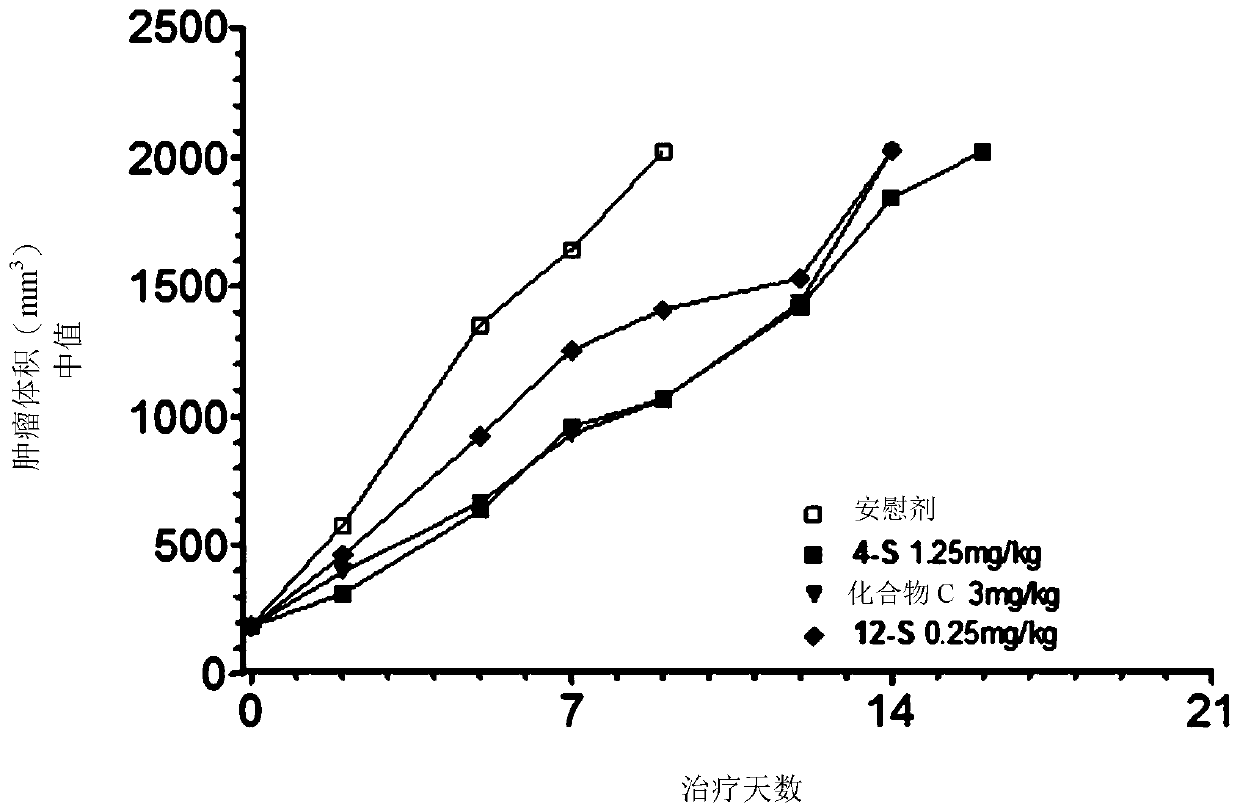
Antitumoral compounds
A compound, selected technology, applied in the field of ascidians
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0830] A)
[0831] To a solution of 1 (0.5 g, 0.80 mmol) in acetic acid (20 mL, 0.04 M) was added L-tryptophanol (2-S) (533 mg, 3.0 mmol, Sigma-Aldrich). The reaction mixture was stirred at 23°C for 16 hours, then the acetic acid was evaporated. Add saturated NaHCO 3 aqueous solution with CH 2 Cl 2 Extract the mixture. through anhydrous Na 2 SO 4 The combined organic layers were dried, filtered and concentrated in vacuo. Flash column chromatography (hexane:EtOAc, 1:1) gave compound 3-S (616 mg, 97%) and 3a-S (12 mg, 2%).
[0832] 3-S
[0833] R f =0.50 (hexane:EtOAc, 1:1).
[0834] 1 H NMR (300MHz, CDCl 3 ): δ7.71(s,1H),7.36(dd,J=7.9,1.0Hz,1H),7.27(dd,J=8.2,0.9Hz,1H),7.13(ddd,J=8.3,7.0,1.2 Hz,1H),7.03(ddd,J=8.0,7.0,1.0Hz,1H),6.62(s,1H),6.26(d,J=1.4Hz,1H),6.04(d,J=1.3Hz,1H ),5.75(s,1H),5.14(dd,J=11.7,1.2Hz,1H),4.60(s,1H),4.41(s,1H),4.36-4.24(m,2H),4.21(d, J=2.7Hz, 1H), 3.82(s, 3H), 3.52(s, 1H), 3.50-3.47(m, 1H), 3.45(dq, J=8.4, 2.2Hz, 1H), 3.35(t, J =10.1Hz,1H...
Embodiment 2
[0854] A)
[0855] To a solution of 1 (0.5 g, 0.80 mmol) in acetic acid (20 mL, 0.04 M) was added D-tryptophanol (2-R) (533 mg, 3.0 mmol, Sigma-Aldrich). The reaction mixture was stirred at 23°C for 16 hours, then the acetic acid was evaporated. Add NaHCO 3 saturated aqueous solution with CH 2 Cl 2 Extract the mixture. Anhydrous Na 2 SO 4 The combined organic layers were dried, filtered and concentrated in vacuo. Flash column chromatography (hexane:EtOAc, 1:1) gave compound 3-R (479 mg, 75%).
[0856] R f =0.44 (hexane:EtOAc, 1:1).
[0857] 1 H NMR (400MHz, CDCl 3 ): δ7.61(s,1H),7.39(d,J=7.8Hz,1H),7.29(d,J=9.6Hz,1H),7.12(t,J=7.3Hz,1H),7.03(t ,J=7.3Hz,1H),6.60(s,1H),6.25(s,1H),6.03(s,1H),5.75(s,1H),5.04(d,J=11.7Hz,1H),4.62 (s,1H),4.37(s,1H),4.32-4.25(m,1H),4.22(d,J=2.7Hz,1H),4.19-4.09(m,1H),3.82(s,3H), 3.77(s,1H),3.64(d,J=9.0Hz,1H),3.49-3.41(m,2H),3.02-2.90(m,2H),2.60-2.52(m,2H),2.45(d, J=14.7Hz, 2H), 2.40(s, 3H), 2.28(s, 3H), 2.22-2.14(m, 2H), 2.18(s, 3H), 2.1...
Embodiment 3
[0866] Example 3 Synthesis of Allyl N-[(R)-(2-amino-3-(1H-indol-3-yl)propyl)]carbamate (9-R)
[0867]
[0868] A)
[0869] CH 3 To the solution of CN (42 mL, 4 mL / mmol) was added di-tert-butyl dicarbonate (4.6 g, 20.8 mmol). The reaction mixture was stirred at 23°C for 3 hours and concentrated in vacuo. Flash column chromatography (CH 2 Cl 2 :CH 3 OH from 99:1 to 85:15) gave 5-R (2.2 g, 73%).
[0870] R f =0.5(CH 2 Cl 2 :CH 3 OH, 9:1).
[0871] 1 H NMR (400MHz, CDCl 3 ):δ8.13(s,1H),7.67(dd,J=7.8,1.1Hz,1H),7.38(dd,J=8.1,1.3Hz,1H),7.29-7.10(m,2H),7.06( s,1H),4.82(s,1H),4.00(s,1H),3.71(dd,J=11.0,3.8Hz,1H),3.62(dd,J=11.0,5.5Hz,1H),3.01(d , J=6.7Hz, 2H), 2.14(s, 1H), 1.44(s, 9H).
[0872] B)
[0873] To 5-R (2.4g, 8.2mmol) in CH 2 Cl 2 (50 mL, 6 mL / mmol) were added phthalimide (2.7 g, 18.2 mmol), triphenylphosphine (4.8 g, 18.2 mmol), and the mixture was cooled at 0°C. CH with addition of diethyl azodicarboxylate 2 Cl 2 solution (25 mL, 3 mL / mmol) for 15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com