GIP and GLP-1 double-agonistic polypeptide compound and pharmaceutically acceptable salt and application thereof
A GLP-1, peptide compound technology, applied in hormone peptides, organic chemistry, hormone receptors, etc., can solve problems such as prolonging the half-life of peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
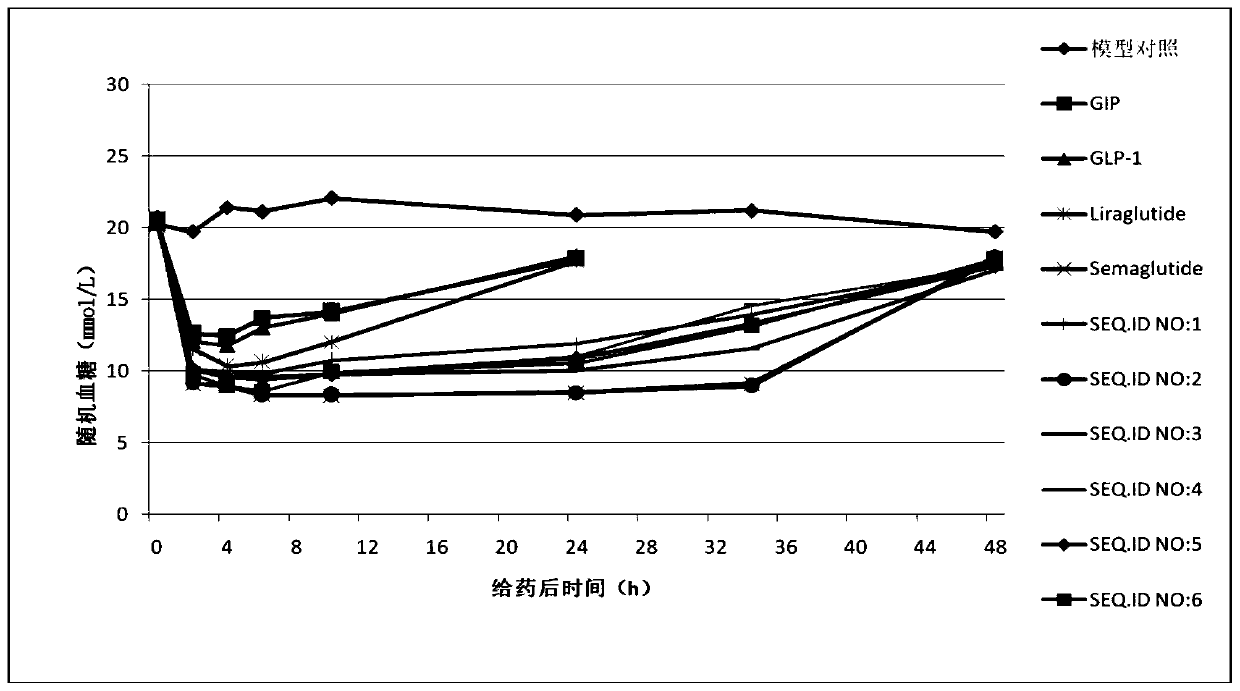
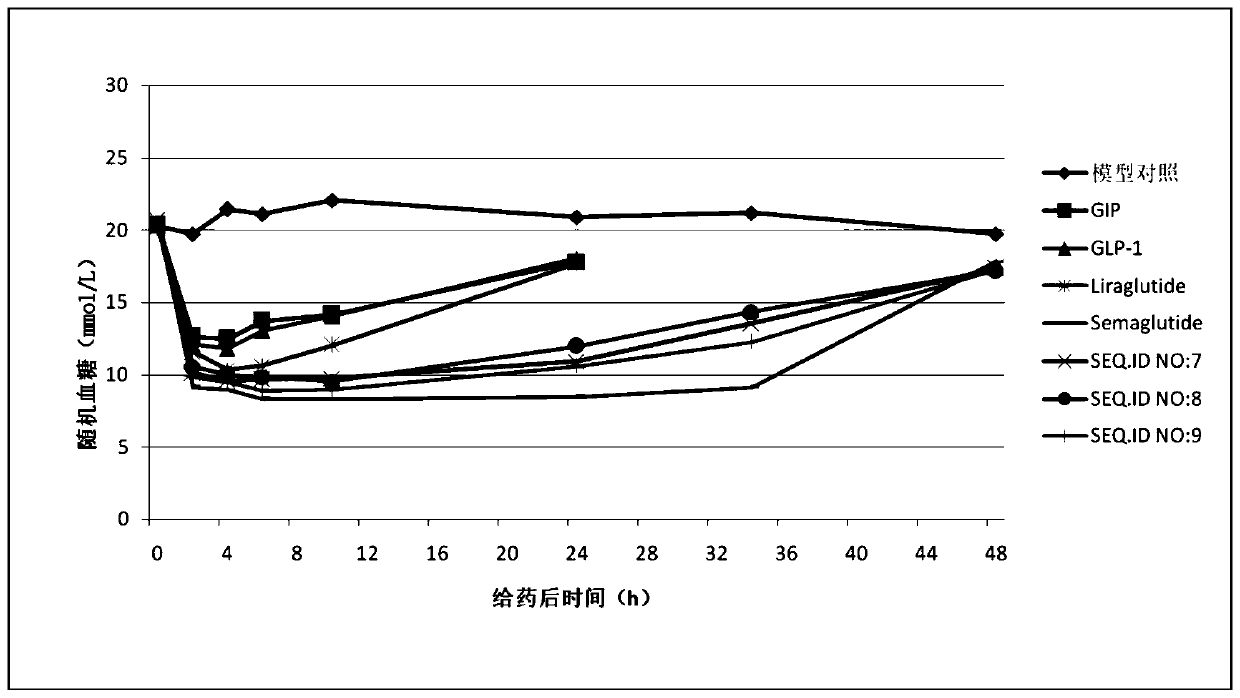
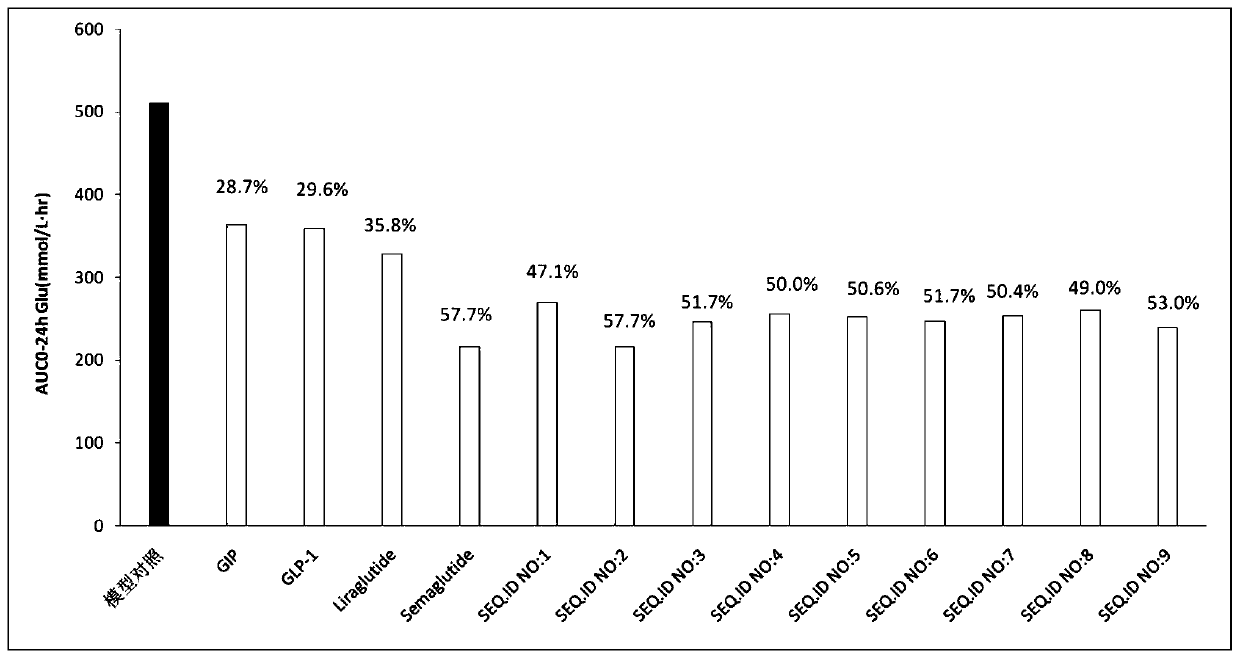
Image
Examples
Embodiment 1
[0090] Embodiment 1, the synthesis of SEQ ID NO: 1 polypeptide compound:
[0091] YVEGTFTSDYSIVLDKIAQK(HOOC-(CH 2 ) 16 -CO-γ-Glu-AEEA-AEEA)AFVQWLIAGGPSSGAPPPS-NH 2
[0092] 1. Synthesis of peptide chains
[0093] 1.1 Resin swelling
[0094] Weigh 10g of Fmoc-Rink Amide AM Resin (degree of substitution 0.35mmol / g), swell with 100mL of DCM for 30min, filter to remove DCM, then swell with 100mL of DMF for 30min, rinse with DMF and 100mL of DCM respectively.
[0095] 1.2 Synthesis of Fmoc-Ser(tBu)-Rink Amide AM Resin
[0096]Dissolve Fmoc-Ser(tBu)-OH (8mmol), HOBT (16mmol) and DIC (16mmol) in 100mL of DMF, then add this solution to the resin obtained in the previous step to react for 2 hours, and filter the reaction solution after completion. The resin was washed 3 times with 100 mL each of DCM and DMF.
[0097] 1.3 Removal of Fmoc protecting group
[0098] Add 25% piperidine / DMF (V / V) solution containing 0.1M HOBt to the washed resin to remove Fmoc, and wash the resin 3 t...
Embodiment 2
[0108] Embodiment 2, the synthesis of SEQ ID NO:2 polypeptide compound:
[0109] YXEGTFTSDYSIXLDKIAQK(HOOC-(CH 2 ) 16 -CO-γ-Glu-AEEA-AEEA)AFVQWLIAGGPSSGAPPPS-NH 2
[0110]
[0111] The synthesis method was the same as in Example 1, and the collected solution was freeze-dried to obtain 1.45 g of pure product.
Embodiment 3
[0112] Embodiment 3, the synthesis of SEQ ID NO:3 polypeptide compound:
[0113] YXEGTFTSDYSIXLDKIAQK(HOOC-(CH 2 ) 16 -CO-γ-Glu-AEEA-AEEA)AFVQWLIAGGPSSGAPPPS-NH 2
[0114]
[0115] The synthesis method was the same as in Example 1, and the collected solution was lyophilized to obtain 1.51 g of pure product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com