Purpose of mitoxantrone hydrochloride liposome for treating non-hodgkin lymphoma
A liposome, lymphoma technology, applied in the field of anti-tumor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 Mitoxantrone liposome is used alone to treat DLBCL
[0058] standard constrain:
[0059]Our company has conducted a phase II clinical study of mitoxantrone liposomes in the treatment of DLBCL and peripheral T / NK cell lymphoma. The inclusion criteria for this study are as follows:
[0060] 1) Volunteer to be tested and sign the informed consent form;
[0061] 2) Aged 18-75 years old, gender is not limited;
[0062] 3) ECOG score 0-2;
[0063] 4) Expected survival time ≥ 3 months;
[0064] 5) Non-Hodgkin's lymphoma with diffuse large B cells and peripheral T / NK cells confirmed by histopathology, and peripheral T / NK cell lymphoma is limited to the following types: Peripheral T-cell lymphoma (not specified) , angioimmunoblastic T-cell lymphoma, ALK+ systemic anaplastic large T-cell lymphoma, ALK-systemic anaplastic large T-cell lymphoma, extranodal NK / T-cell lymphoma, nasal type, enteropathy-associated T-cell lymphoma tumor, primary hepatosplenic γδT-cell ...
Embodiment 2
[0086] Example 2 The adverse reaction analysis of mitoxantrone liposome used alone in the treatment of DLBCL:
[0087] Security Analysis:
[0088] Analyzing the adverse reactions of the above-mentioned DLBCL subjects, the highest incidence of adverse reactions was hematological toxicity.
[0089] In terms of hematological toxicity, 31.4% of the subjects experienced grade 3 or higher white blood cell count reduction, 28.6% of the subjects experienced grade 3 or higher neutropenia, and 5.7% of the subjects experienced grade 3 or higher thrombocytopenia. This is much lower than the hematological toxicity recorded in the literature (Cancer Sci January 2007, vol.98) analyzed in the background technology (in this study, 18 cases (60%) had 3-4 grade hematological toxicity. 8 cases (27%) had grade 4 neutropenia despite the use of leukocyte-raising drugs (G-CSF). Five patients (16.7%) had grade 3-4 thrombocytopenia). Among the non-hematological toxicities, 5.7% of the subjects had gr...
Embodiment 3
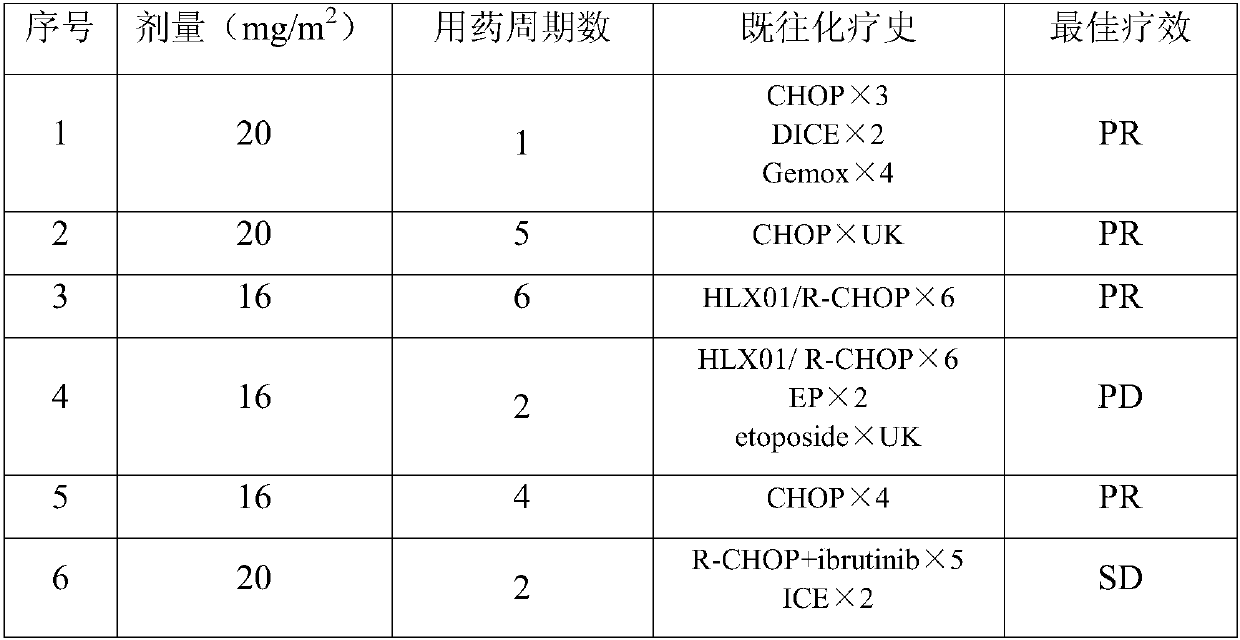
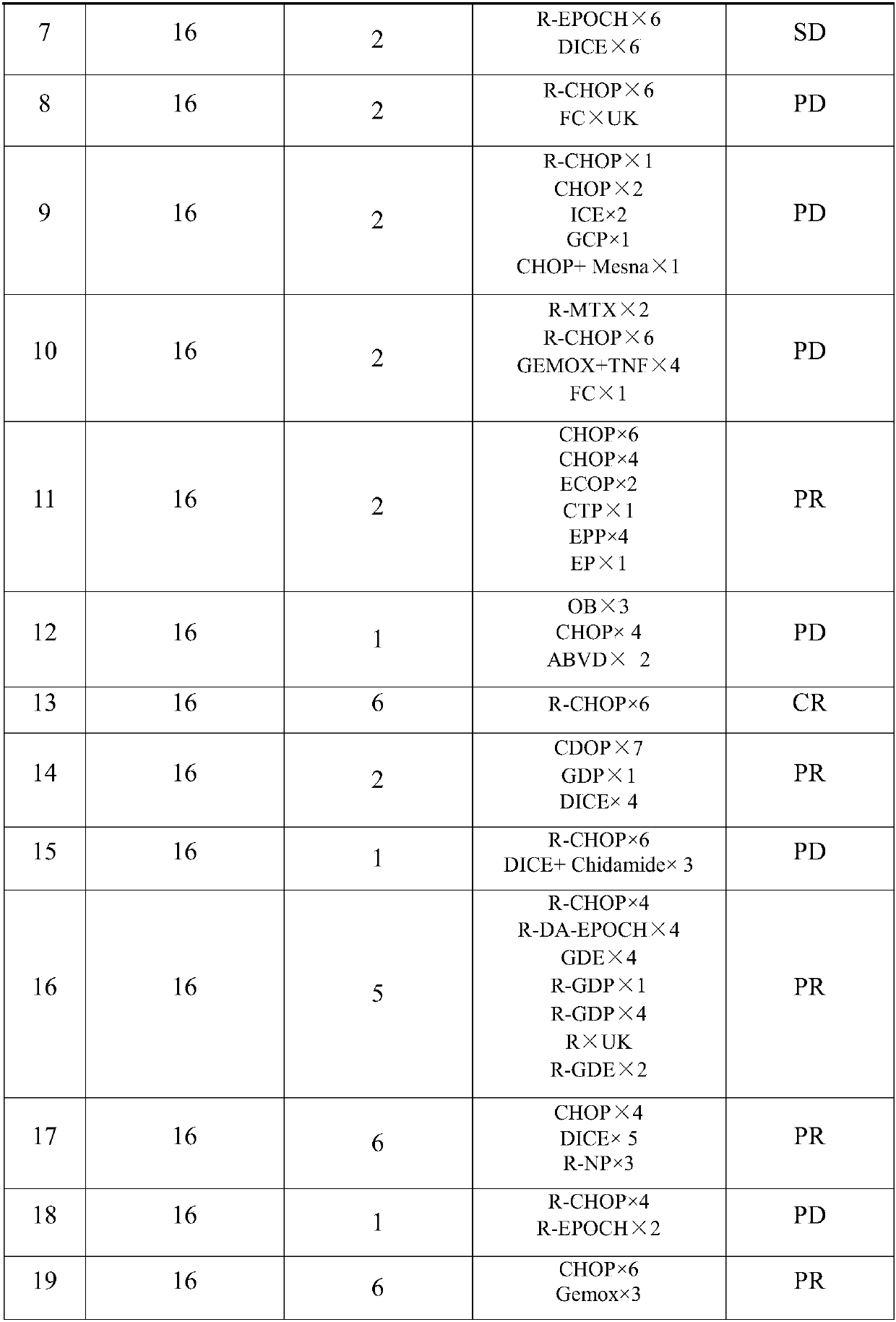
[0090] Embodiment 3 Mitoxantrone liposome is used alone to treat PTCL
[0091] The inclusion criteria, administration method, safety and curative effect evaluation are the same as in Example 1.
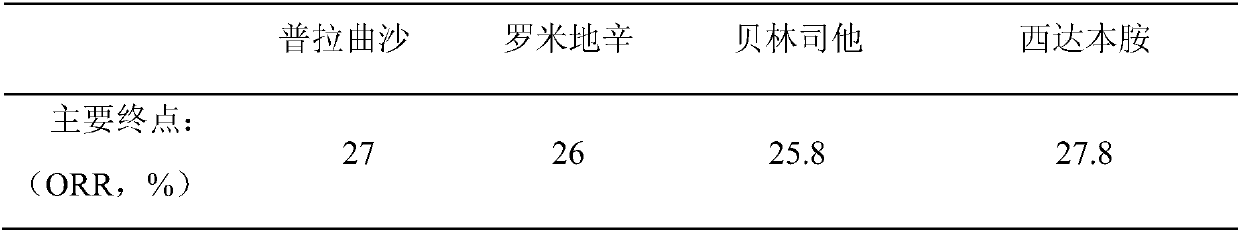
[0092] The mitoxantrone liposome developed by our company is positioned as the second-line treatment in the treatment of PTCL. The ORR of this product in clinical research is 53.8%, which is comparable to the currently recommended second-line HDAC inhibitors, including pralatrexate and romidepsin. Compared with HDAC inhibitors, belinostat and chidamide (HDAC inhibitors are oral preparations, administered once a day, ORR is lower than 30%), it is much higher than that of HDAC inhibitors. Patients who achieve remission in a short period of time can undergo bone marrow transplantation in time.
[0093] Mitoxantrone liposome is an injection, the dosage is 14, 16, 20mg / m 2 or 24mg / m 2 , administered once every 28 days. Subjects received an average of 3.6±1.8 cycles of treatment.
[00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com