Method for preparing perovskite-type composite oxide high entropy ceramic powder by ion compensation mixture coprecipitation
A composite oxide and perovskite technology, applied in the field of lead-free dielectric ceramic materials, can solve the problems of component segregation and the deviation of the final product from the stoichiometric ratio, and achieve the effect of avoiding precipitation and decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
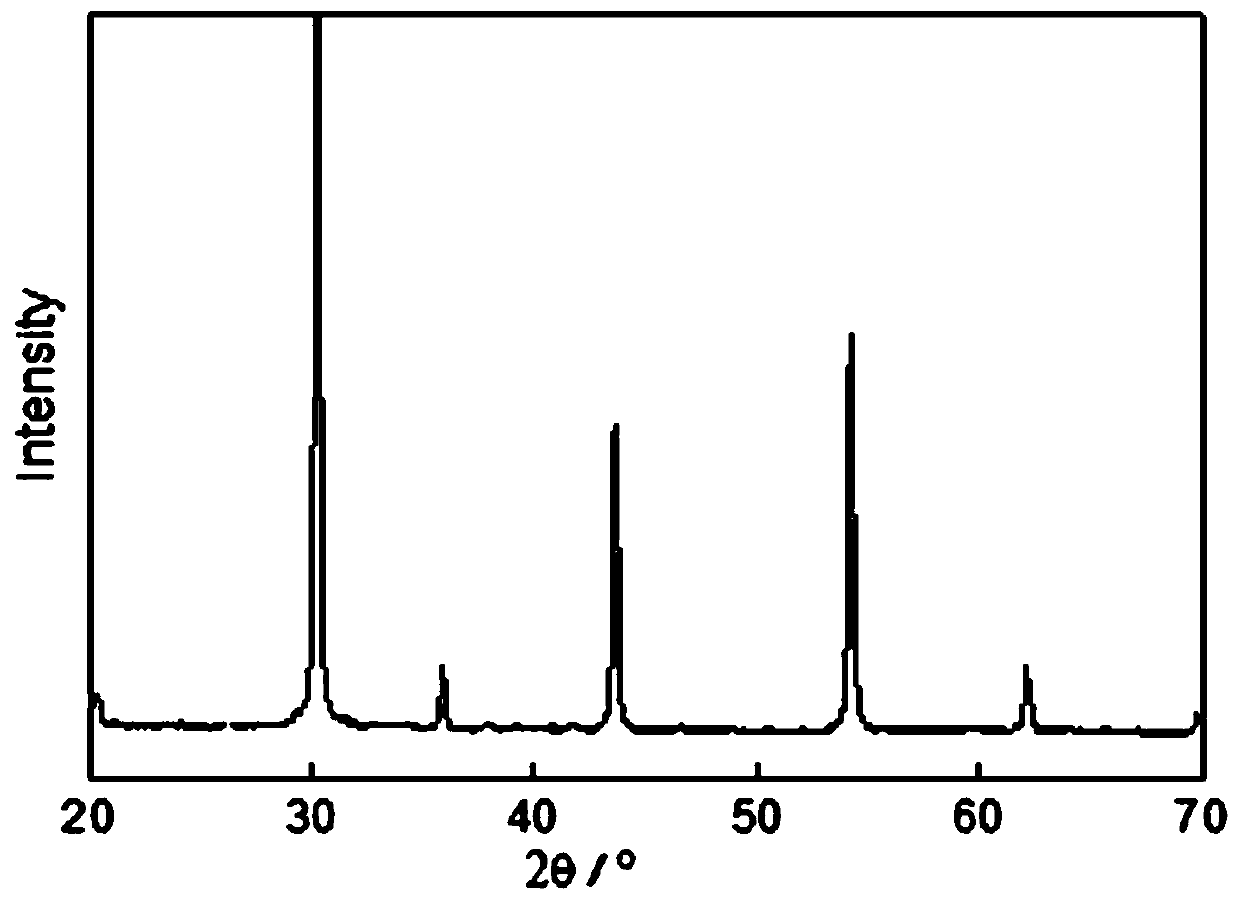
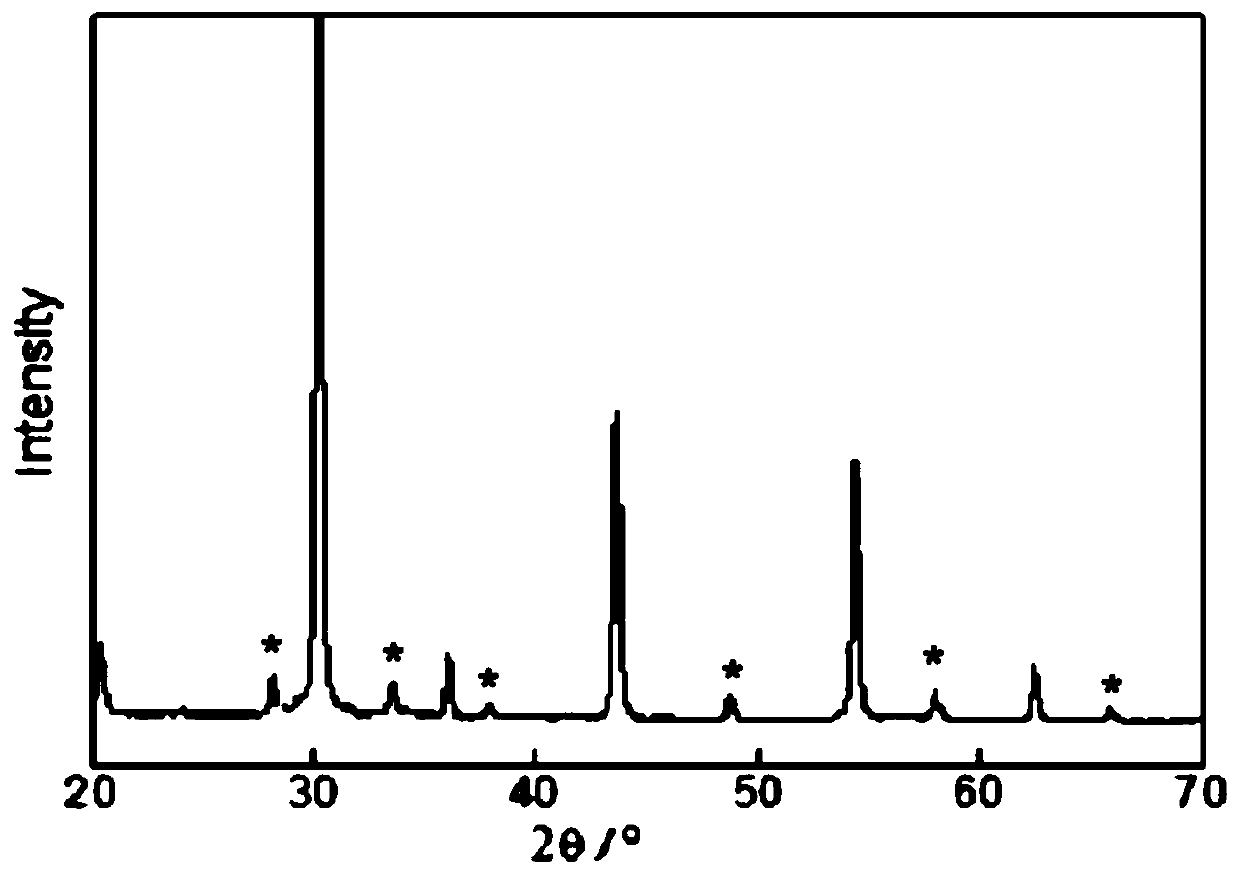
Image
Examples
Embodiment 1
[0024] (1) with analytically pure BaCl 2 ·H 2 O, ZrCl 4 、TiCl 4 , SnCl 2 , niobium oxalate and high-purity YCl 3 ·6H 2 O, CeCl 3 ·7H 2 O or ErCl 3 ·6H 2 O is raw material, according to the molar ratio of Zr, Sn, Ti, Nb, M is 1:1.1:1.05:1:1, Ba:(Zr 0.2 sn 0.2 Ti 0.2 Nb 0.2 m 0.2) in a molar ratio of 1.05:1, wherein M is one of Y, Ce or Er.
[0025] (2) Dissolve analytically pure concentrated hydrochloric acid in deionized water to prepare a 0.1mol / L hydrochloric acid solution; 4 and SnCl 2 respectively added to hydrochloric acid solution to prepare TiCl 4 and SnCl 2 hydrochloric acid solution, hydrochloric acid is added dropwise to the solution, and the pH value is adjusted to 2-4; the BaCl 2 ·H 2 O, ZrCl 4 , niobium oxalate and MCl 3 Dissolve in deionized water, stir for 60 minutes and slowly add TiCl 4 and SnCl 2 hydrochloric acid solution, continue stirring for 30 minutes to obtain a mixed salt solution with a concentration of 0.5 mol / L, add hydrochlor...
Embodiment 2
[0031] (1) with analytically pure SrCl 2 ·6H 2 O, ZrCl 4 , SnCl 2 、TiCl 4 , HfCl 4 High purity YCl 3 ·6H 2 O or MnCl 2 ·6H 2 O is raw material, according to the molar ratio of Zr, Sn, Ti, Hf, M is 1:1.1:1.05:1.10:1, Sr:(Zr 0.2 sn 0.2 Ti 0.2 f 0.2 m 0.2 ) in a molar ratio of 1.05:1, where M is Mn or Y.
[0032] (2) Dissolve analytically pure concentrated hydrochloric acid in deionized water to prepare a 0.1mol / L hydrochloric acid solution; 4 , SnCl 2 and HfCl 4 respectively added to hydrochloric acid solution to prepare TiCl 4 , SnCl 2 and HfCl 4 hydrochloric acid solution, hydrochloric acid is added dropwise to the solution, and the pH value is adjusted to 2-4; the BaCl 2 ·H 2 O, ZrCl 4 , and MCl 3 Dissolve in deionized water, stir for 60 minutes and slowly add TiCl 4 , SnCl 2 and HfCl 4 hydrochloric acid solution, and continue to stir for 30 minutes to obtain a mixed salt solution with a concentration of 0.5 mol / L. Hydrochloric acid is added dropwise ...
Embodiment 3
[0038] (1) with analytically pure SrCl 2 ·6H 2 O, BaCl 2 , ZrCl 4 、TiCl 4 , SnCl 2 , niobium oxalate, high-purity YCl 3 ·6H 2 O is the raw material, according to the chemical formula Ba of the target product 0.5 Sr 0.5 (Zr 0.2 sn 0.2 Ti 0.2 Nb 0.2 Y 0.2 )O 3 , according to the molar ratio of Zr, Sn, Ti, Nb, Y is 1:1.1:1.05:1:1, (Ba 0.5 Sr 0.5 ): (Zr 0.2 sn 0.2 Ti 0.2 Nb 0.2 Y 0.2 ) in a mol ratio of 1.02:1 for proportioning.
[0039] (2) Dissolve analytically pure concentrated hydrochloric acid in deionized water to prepare a 0.1mol / L hydrochloric acid solution; 4 and SnCl 2 respectively added to hydrochloric acid solution to prepare TiCl 4 and SnCl 2 hydrochloric acid solution, hydrochloric acid is added dropwise to the solution, and the pH value is adjusted to 2-4; the SrCl 2 ·6H 2 O, BaCl 2 ·H 2 O, ZrCl 4 , niobium oxalate and YCl 3 ·6H 2 O was dissolved in deionized water, and TiCl was slowly added to it after stirring for 60 min 4 and SnCl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com