Novel ambergris and/or indole-like compositions of odoriferous substances
A fragrance and composition technology, applied in the directions of essential oils/fragrances, detergent composition fragrances, formulations of fragrance preparations, etc., can solve the problem of difficulty in expressing the odor of ambergris, and achieve improved olfactory impression, high odor intensity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
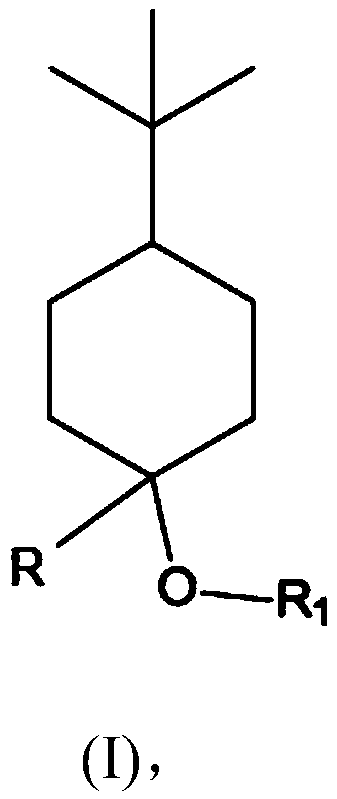
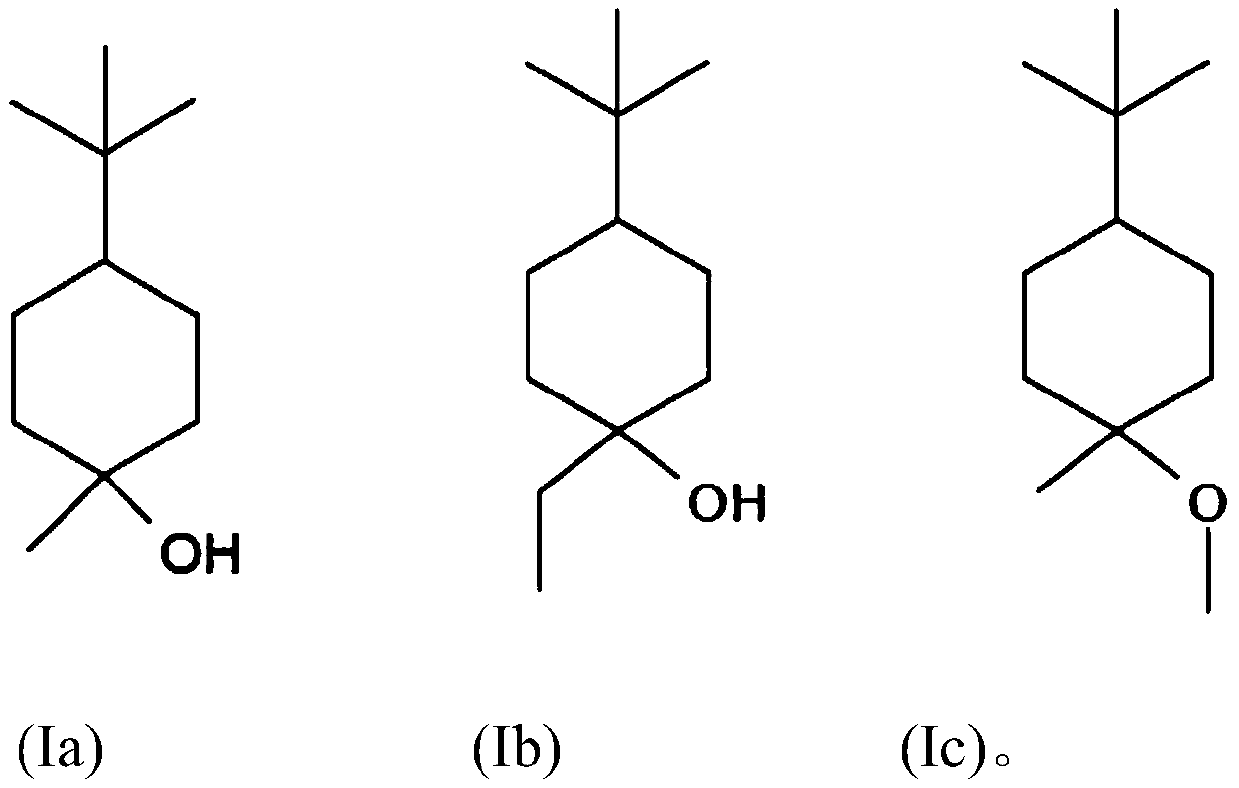
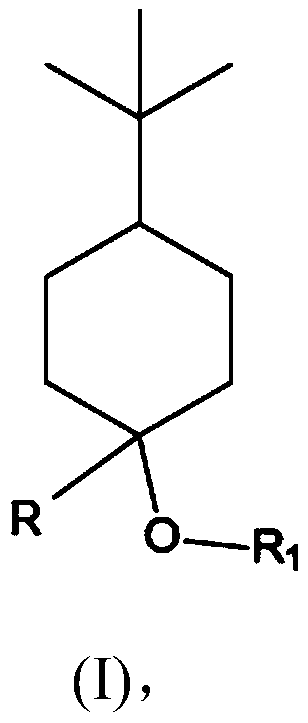
[0124] Example 1: Synthesis of 4-tert-butyl-1-methyl-cyclohexanol (Grignard reaction)
[0125] 200 ml of methylmagnesium chloride solution (3M in THF) and 100 ml of THF were placed in a stirrer with a magnetic stirrer under a nitrogen atmosphere. Subsequently, 83.2 g (0.54 mol) of 4-tert-butylcyclohexanone dissolved in 200 ml of THF was added dropwise at 20° C. to 25° C. within 1 hour to 2 hours. The mixture was further stirred at room temperature for 2 to 3 hours. The deposit was poured into a cold saturated ammonium chloride solution at 0° C. to 5° C., then extracted by shaking with MTBE, and the organic phase was washed with soda solution and then with NaCl solution. After evaporation, 84.6 g of crude product were obtained.
[0126] The material is distilled in a small column.
[0127] Yield: 77.8 g (84.7% of theory)
Embodiment 2
[0128] Embodiment 2: the synthesis (etherification) of 4-tert-butyl-1-methyl-cyclohexanol methyl ether
[0129] In a stirrer with a magnetic stirrer, a solution consisting of 42.5 g (0.25 mol) of 4-tert-butyl-1-methyl-cyclohexanol in 100 ml of anhydrous THF at 20 °C was brought under nitrogen atmosphere 0.3 mol of 60% sodium hydride in anhydrous THF was added dropwise. It was then stirred for a further 5 hours at room temperature. Subsequently, 42.6 g (0.3 mol) of methyl iodide in 100 ml of anhydrous THF were added dropwise at room temperature within 30 minutes. It was then stirred for a further 2 hours at room temperature. The reaction mixture was carefully mixed with 250 ml of ice-cold water under cooling, and after separation of the aqueous phase, it was washed neutrally again twice with 50 ml of saturated saline solution each. After distilling off the solvent, the residue was distilled in a small column.
[0130] Yield: 28.8 g (62.8% of theory)
[0131] The same auxil...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com