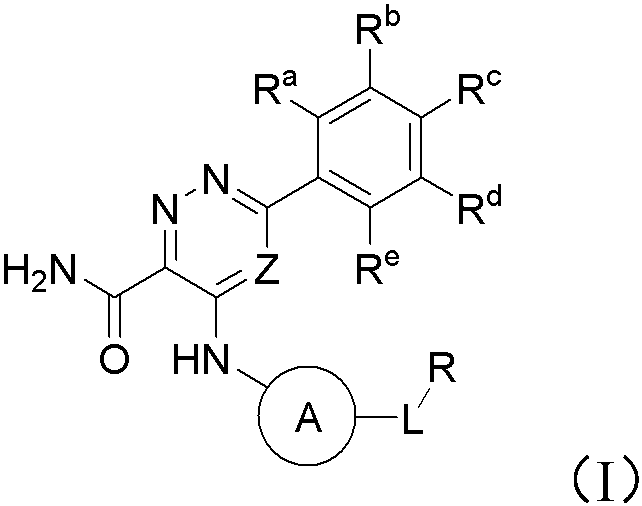
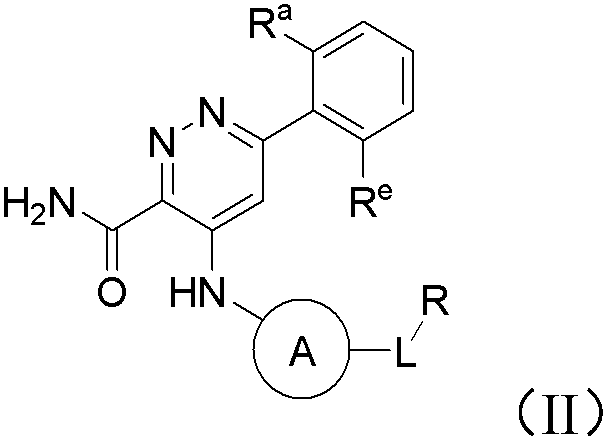
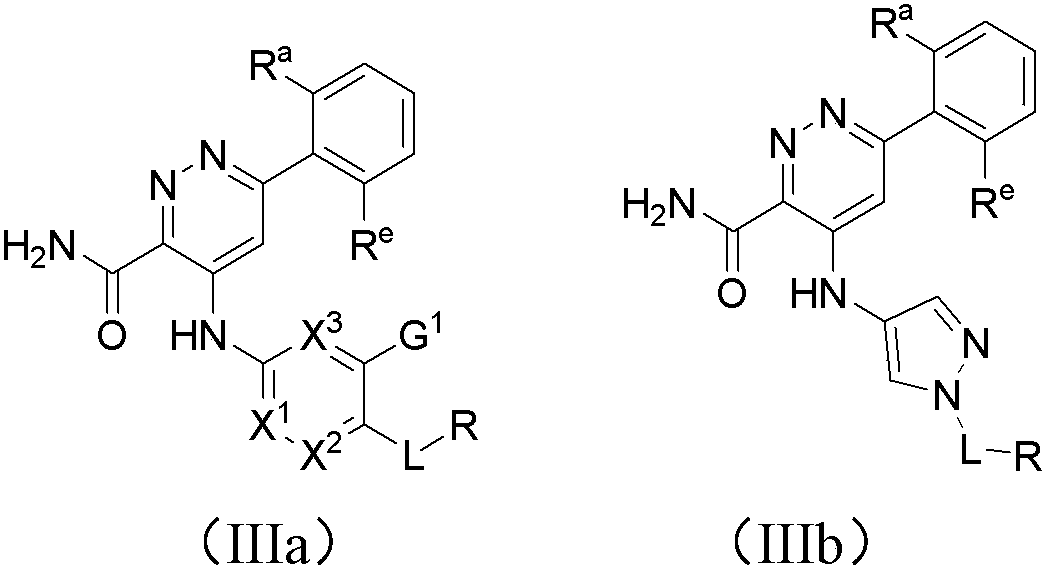
Pyridazine-3-carboxamide compound and preparation method and application thereof in medicine
A technology of compounds and mixtures, applied in the fields of active ingredients of heterocyclic compounds, organic chemistry, pharmaceutical formulations, etc., can solve problems such as difficulties in research and development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0221] 6-(2,6-Difluorophenyl)-4-((4-ethoxyphenyl)amino)pyridazine-3-carboxamide
[0222]
[0223] first step
[0224] Methyl 6-chloro-4-((4-ethoxyphenyl)amino)pyridazine-3-carboxylate
[0225] Compound 4,6-dichloropyridazine-3-carboxylic acid methyl ester 1a (206mg, 1.00mmol), 4-ethoxyaniline (205mg, 1.50mmol) and diisopropylethylamine (387mg, 3.00mmol ) was dissolved in acetonitrile (3 mL), then heated to 85° C. and stirred for 2 hours in a microwave reactor. After cooling to room temperature, the solvent was removed under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate=50 / 1 to 10 / 1) to obtain the target product 6-chloro-4-((4-ethoxy Phenyl)amino)pyridazine-3-carboxylic acid methyl ester 1b (240 mg, yellow oil), yield: 78%.
[0226] MS m / z(ESI): 308[M+1]
[0227] second step
[0228] 6-(2,6-Difluorophenyl)-4-((4-ethoxyphenyl)amino)pyridazine-3-carboxylic acid methyl ester
[0229] Compound 6-chloro-4-(...
Embodiment 8
[0244] 6-(2,6-Difluorophenyl)-4-((4-(methylsulfonyl)phenyl)amino)pyridazine-3-carboxamide
[0245]
[0246] first step
[0247] Methyl 6-chloro-4-((4-(methylsulfonyl)phenyl)amino)pyridazine-3-carboxylate
[0248] The compound 4,6-dichloropyridazine-3-carboxylate methyl ester 1a (206 mg, 1.00 mmol) and 4-methanesulfonylaniline (342 mg, 2.00 mmol) were dissolved in ethanol (3 mL), and then dissolved in a microwave reactor Heat to 80°C and stir for 1 hour. After cooling to room temperature, the solvent was removed under reduced pressure, and the residue was purified by silica gel column chromatography (petroleum ether / ethyl acetate=50 / 1 to 1 / 1) to obtain the target product 6-chloro-4-((4-(methyl Methylsulfonyl)phenyl)amino)pyridazine-3-carboxylate 8a (220 mg, yellow solid), yield: 64%.
[0249] MS m / z(ESI): 342[M+1]
[0250] Step 2 to Step 4
[0251] 6-(2,6-Difluorophenyl)-4-((4-(methylsulfonyl)phenyl)amino)pyridazine-3-carboxamide
[0252] Synthesize the target product ...
Embodiment 9
[0256] 4-((4-cyanophenyl)amino)-6-(2,6-difluorophenyl)pyridazine-3-carboxamide
[0257]
[0258] Example 9 was synthesized according to the synthesis steps of Example 8, but in the first step, 4-cyanoaniline was used instead of 4-methylsulfonylaniline.
[0259] MS m / z(ESI): 352[M+1]
[0260] 1 H NMR (400MHz, DMSO-d 6 )δ11.24(s, 1H), 8.87(s, 1H), 8.15(s, 1H), 7.84(d, J=7.8Hz, 2H), 7.67-7.42(m, 4H), 7.30(d, J = 7.6Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com