Improved therapeutic T cell
A therapeutic, cell-based technology, applied in the direction of genetically modified cells, animal cells, cells modified by introducing foreign genetic material, etc., can solve problems such as difficult to meet clinical needs and low co-expression efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1. Optimization of lentiviral vectors for expressing CAR
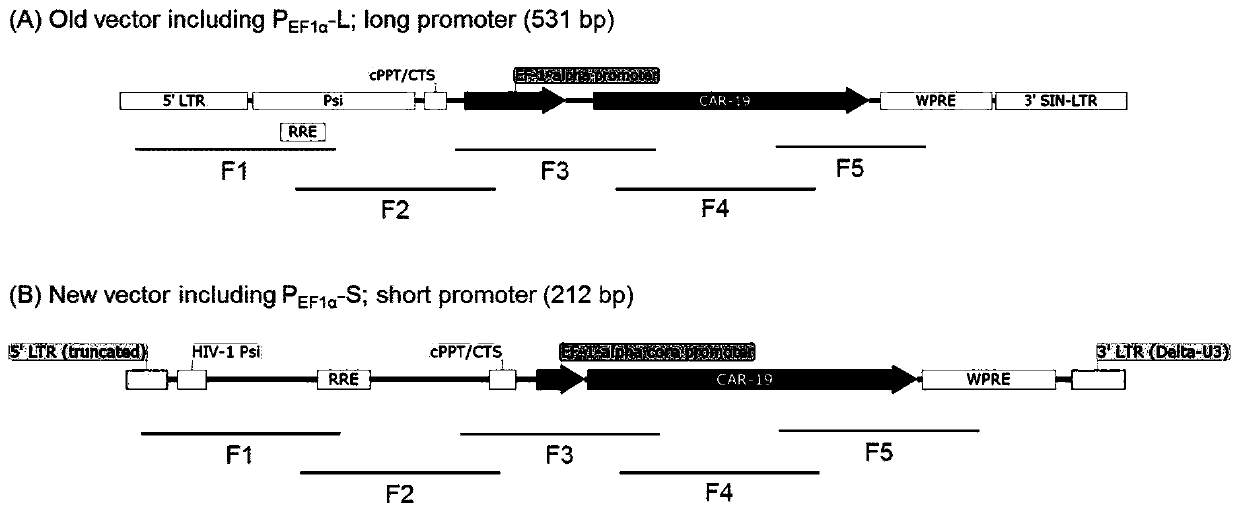
[0071] The lentiviral vector used to transduce CAR should contain the desired CAR transgene and be able to express it in the cell. Two third-generation lentiviral vectors for CAR expression were designed, namely the old vector pPVLV1 ( figure 1 A) and the new vector pPVLV2 ( figure 1 B). pPVLV1 contains the 531 bp long human elongation factor 1 alpha (EF1 alpha) promoter and pPVLV1 contains the 212 bp truncated human EF1 alpha promoter. The various elements contained in the two vectors and their descriptions are found in Table 1 below.
[0072] The CAR to be expressed in the embodiment of the present application comprises scFv targeting CD19, hinge and transmembrane domain of human CD8, intracellular domain 4-1BB and CD3ζ. The amino acid of the CAR targeting CD19 is shown in SEQ ID NO:16, and the nucleotide sequence is shown in SEQ ID NO:8.
[0073] Table 1. Related elements on pPVLV1 and pPVLV2 len...
Embodiment 2
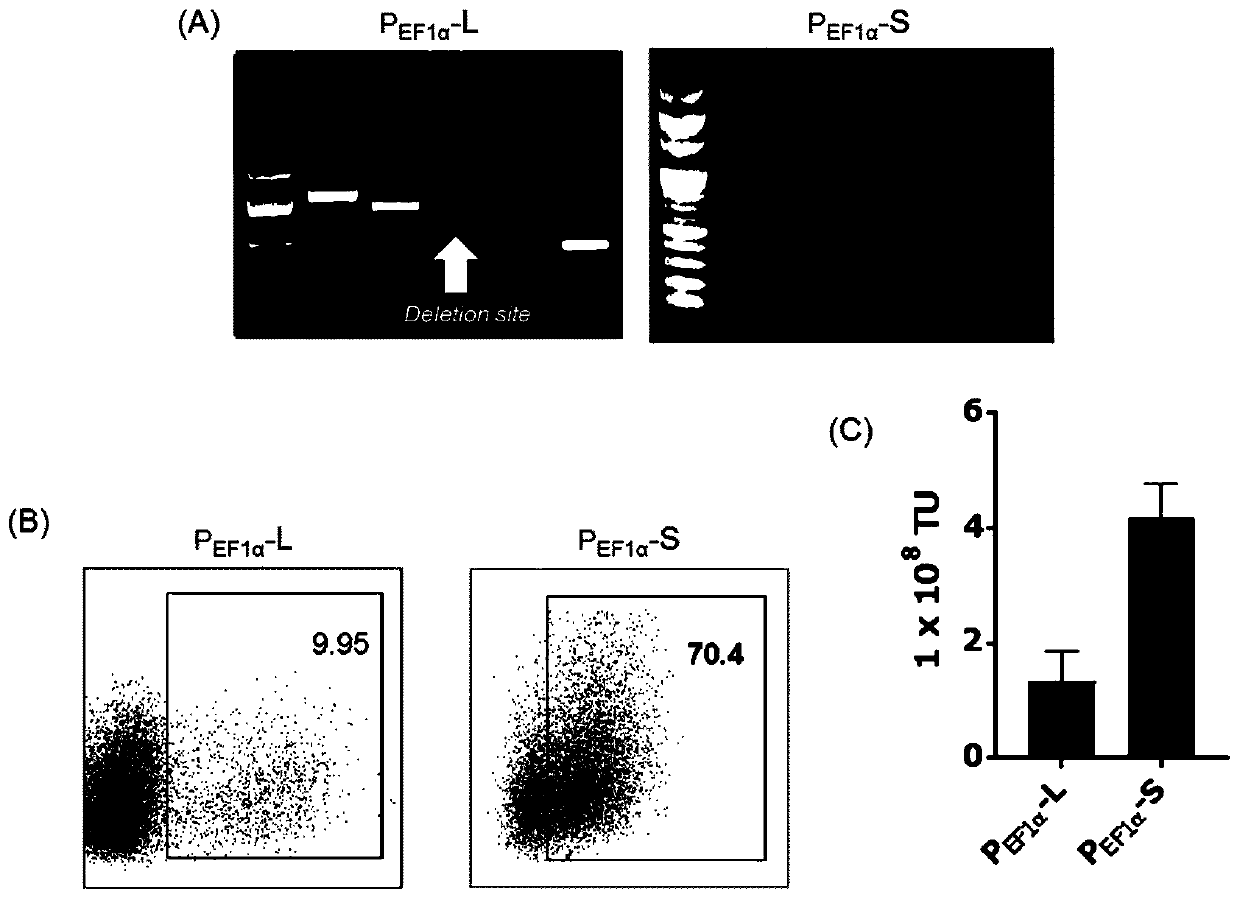
[0082] Example 2, promoter affects the transduction and expression of CAR gene
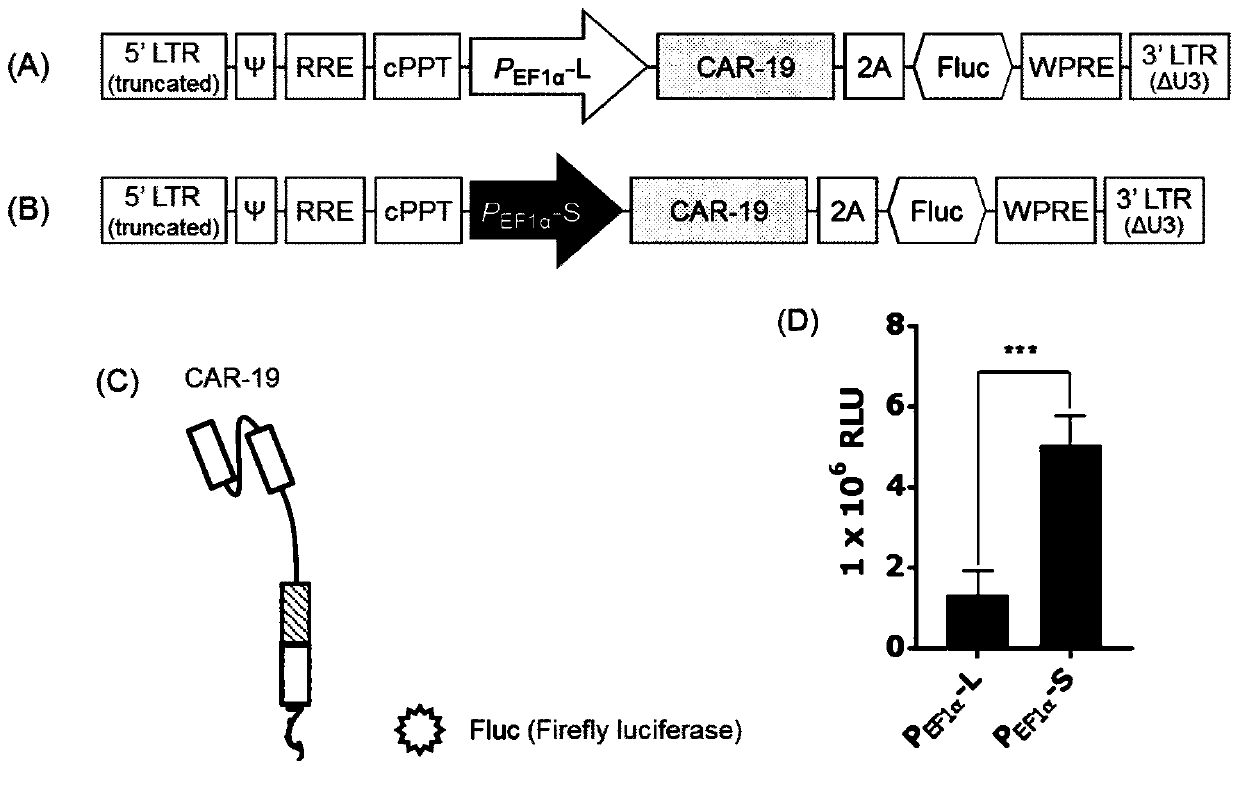
[0083] In order to further demonstrate the influence of different promoters, a pPVLV2-based image 3 Two CAR-luciferase reporter vectors, which differ only in the promoter driving the expression of the transgene, in which CAR-19 was cloned upstream of the P2A-Fluc (firefly luciferase) cassette, forming a bicistronic ( image 3 A and B).
[0084] After the vector is transduced into the cell, due to the presence of the coding sequence of the P2A self-cleaving peptide, two molecules, CAR-19 and luciferase, will be expressed in the same cell at a ratio of approximately 1:1, wherein the fluorescence intensity can be Reflects CAR-19 transduction efficiency (see image 3 C schematic diagram). image 3 D shows luciferase activity measured 48 hours after transduction of the two lentiviral vectors into 293T cells. The results show that with P EF1α The fluorescence of cells transduced with lentiviral ve...
Embodiment 3
[0086] Example 3, co-expression of CAR and DNRII in cells
[0087] TGF-β is an important T cell inhibitory factor, which may lead to the weakening or disappearance of the killing effect of therapeutic T cells on target cells. Clinically, TGF-β is widely expressed in a variety of tumor tissues and significantly inhibits the killing activity of tumor-specific T cells on tumor cells, which is an important reason for the failure of immunotherapy. The dominant negative TGF-β receptor type II (DNRII) is a negative regulatory receptor of TGF-β, which can inhibit the inhibitory effect of TGF-β on T cells. The following examples investigate the effect of co-expressing CAR and DNRII in T cells. The amino acid sequence of DNRII is shown in SEQ ID NO:17, and its nucleotide sequence is shown in SEQ ID NO:18.
[0088] First, similar to Example 2, a pPVLV2-based Figure 4 (A and B) Two CAR-19-DNRII vectors that differ only in the promoter driving transgene expression. Among them, CAR-19 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com