Treatment of neuroinflammatory disease
A technology for inflammatory diseases and therapeutic agents, applied in the field of treatment of neuroinflammatory diseases, can solve problems such as involvement, life-threatening side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
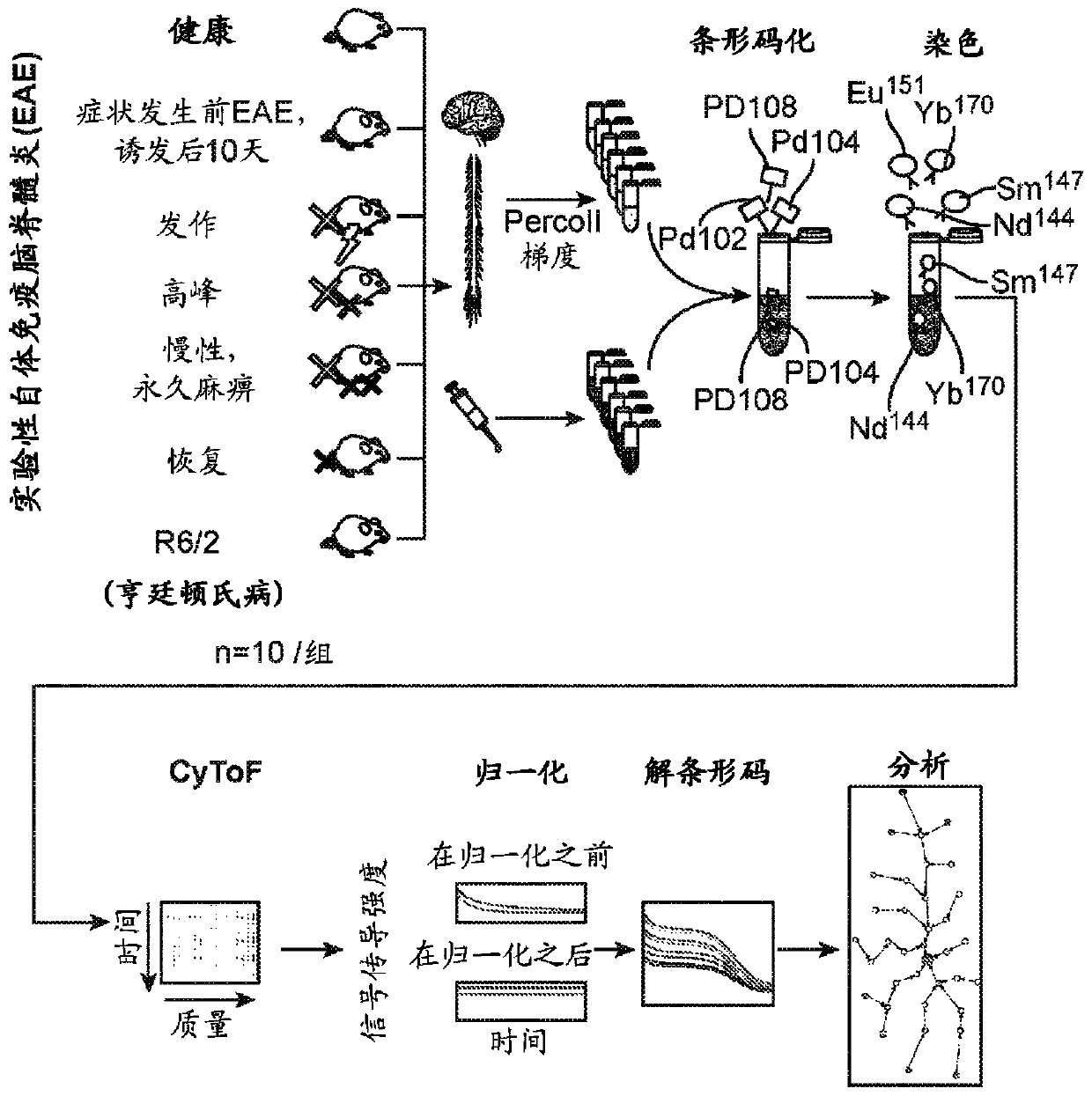
[0120] Single-cell analysis reveals differential molecular signatures in myeloid cells from contrasting neuroinflammation models relative to neurodegeneration models
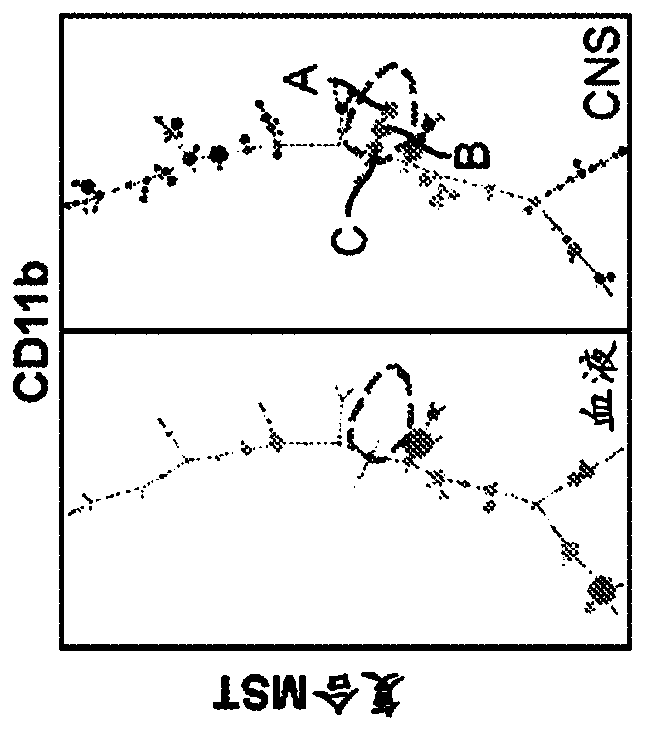
[0121] Two poles are the objects of greater attention in brain pathology: neuroinflammation versus neurodegeneration. Here, we used single-cell mass cytometry (CyToF), which was performed together with unbiased data analysis to compare experimental autoimmune encephalomyelitis ( EAE) mouse model, a system-wide analysis of the immune response in the R6 / 2 mouse model of Huntington's disease (HD) as a neurodegenerative disorder. We identified three populations of myeloid cells that are specific to the central nervous system (CNS) and are present in both neuroinflammatory disorders (EAE) and neurodegenerative disorders (HD). Blood-derived monocytes, the counterparts of CNS-resident myeloid cells, consisted of five subpopulations and were detected in the case of EAE but not in HD. Single-cell analysis revealed larg...
Embodiment 2
[0200] Overview of Myeloid Cell Populations
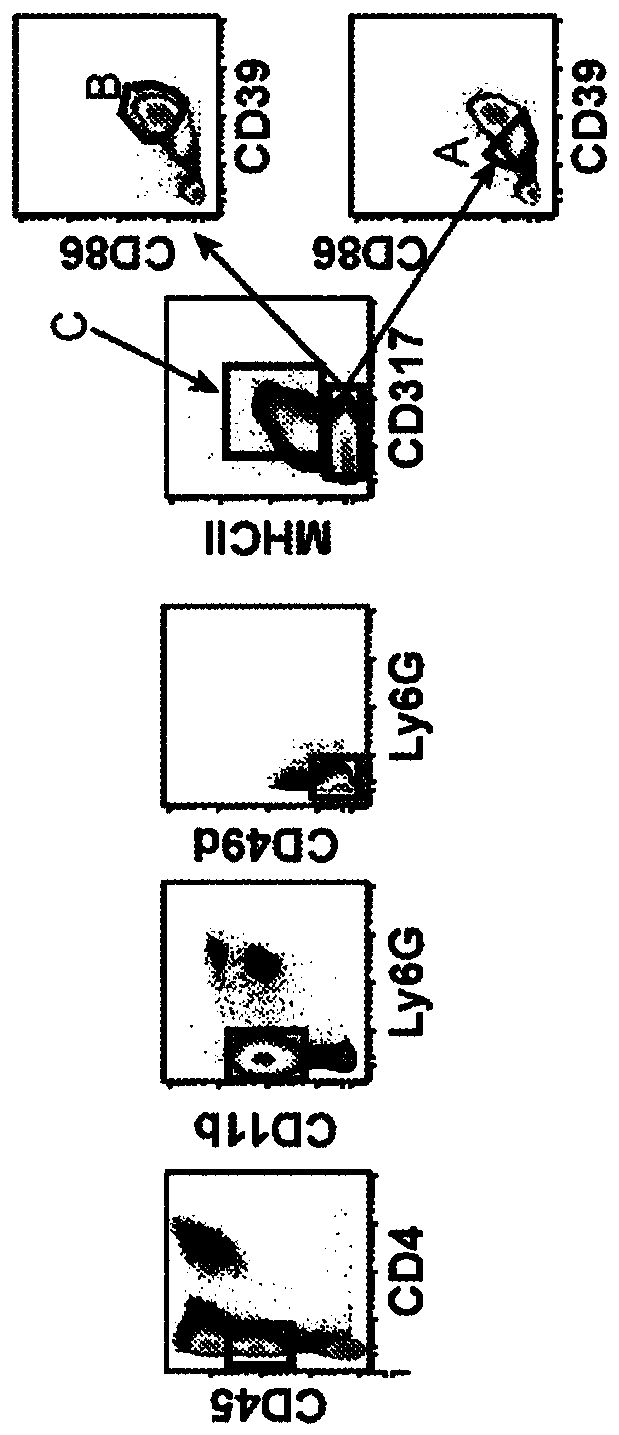
[0201] The phenotypes of the myeloid cell populations discussed herein are summarized in Table 4. Populations A, B and C correspond to microglia. These populations are equivalent to CD45 medium, CD11b+ cells in the human brain.
[0202] In EAE and MS diseases, as well as many inflammatory disorders, there is an infiltration of monocytes from the peripheral blood. We have identified five monocyte populations, referred to herein as D, E, F, H, G, in the central nervous system of EAE mice. In humans, these populations correspond to CD11b+CD14+CD16+ monocytes. Cytokine expression profiles in the context of these populations showed that at the onset or peak of EAE disease, a percentage of these cells expressed multiple inflammatory cytokines compared to a healthy state in which cells expressed only one cytokine ( TNF-α+GMCSF).
[0203] Table 4
[0204]
Embodiment 3
[0206] amyotrophic lateral sclerosis
[0207] Our previous studies and others have demonstrated that microglia are the only myeloid cells in the brain and spinal cord of mSOD1 mice, a murine ALS disease model, and that there is no infiltration of myeloid cells from the peripheral blood (Ajami et al. 2007) Nature Neuroscience 10:1538-1543; Chiu et al. (2013) Cell Reports 4(2):385-401). Furthermore, several studies have demonstrated that microglia are involved in the pathogenesis of ALS, and that limiting expression of mutant SOD in microglia will delay degeneration and prolong the survival of motor mSOD-expressing motor neurons (Clement et al. (2003) Science 302:113-117; Lino et al. (2002) The Journal of Neuroscience 22(12):4825-4832).
[0208] Such as Figure 13 In mice overexpressing human mutant superoxide dismutase 1 (mSOD), a model of murine ALS, there is an increased expression of CD49e in the microglial population at the end-stage of the disease, as shown in . We comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com