A kind of compound preparation of irbesartan hydrochlorothiazide and preparation method thereof
A technology of hydrochlorothiazide and compound preparations, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, drug combinations, etc., can solve the problem of high cost, achieve dissolution guarantee, reduce dosage, and reduce material cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
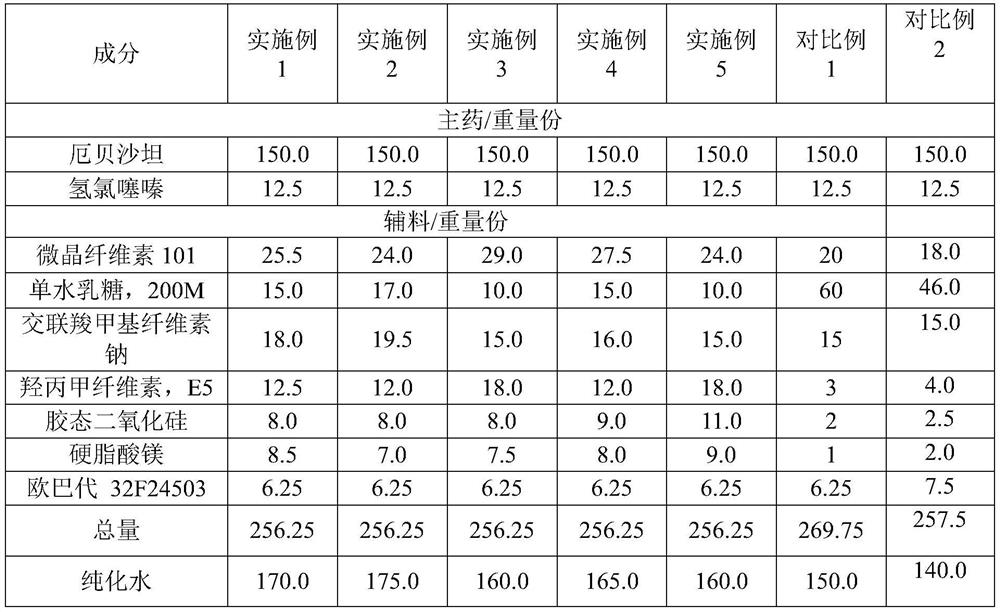
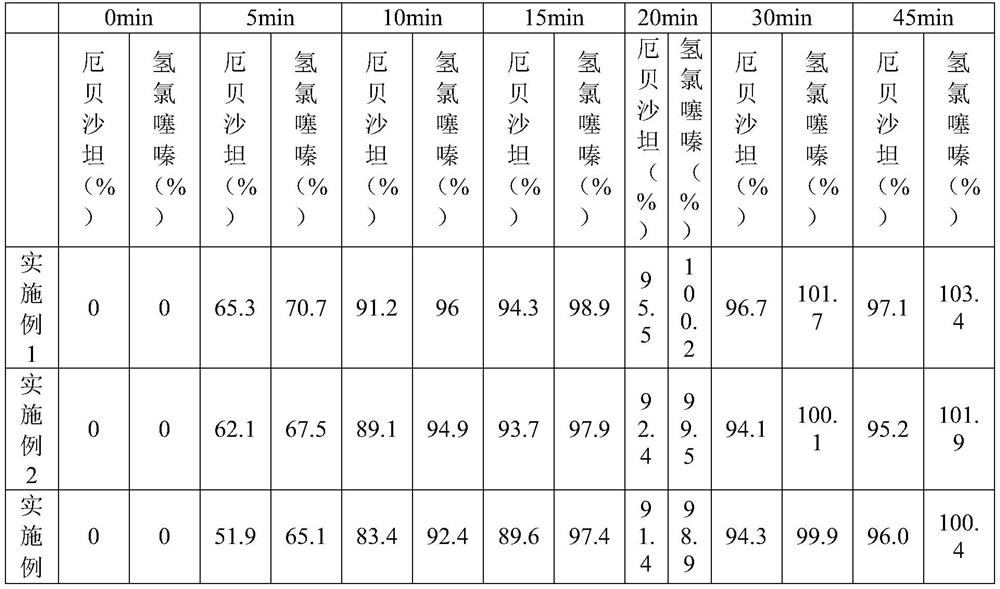
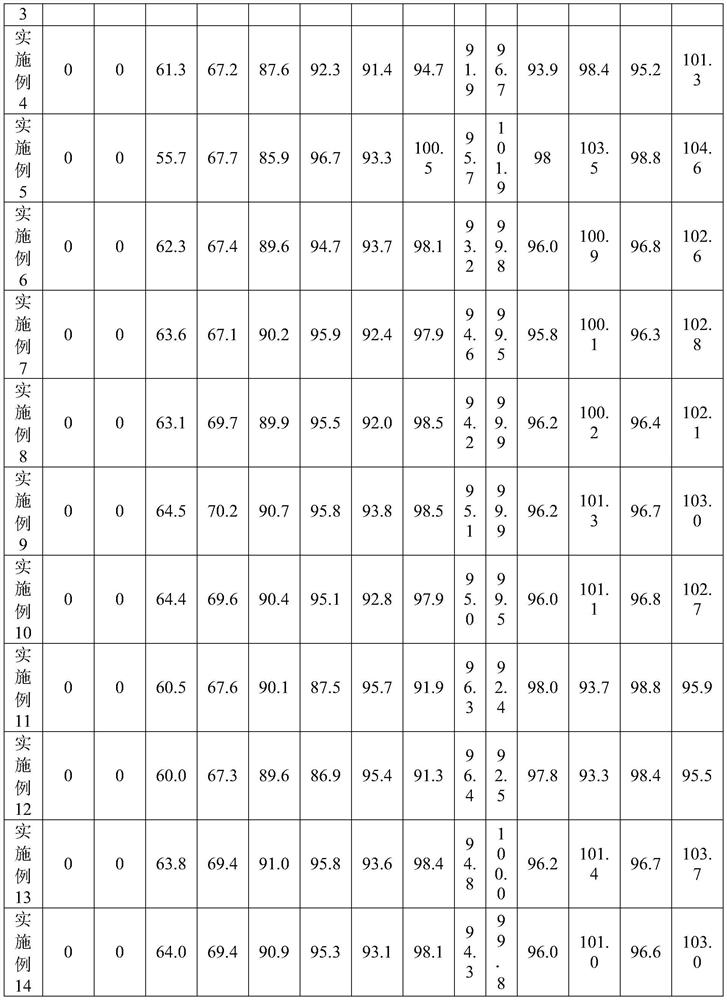
[0028] In one embodiment, the above-mentioned preparation method includes: step S1, adding the prescribed amount of irbesartan, the prescribed amount of hydrochlorothiazide, the prescribed amount of microcrystalline cellulose, the prescribed amount of lactose monohydrate, and part of the prescribed amount of colloidal Silicon dioxide is mixed with croscarmellose sodium of part prescription quantity to obtain the first mixture, preferably part of the prescription quantity of colloidal silicon dioxide is 40-60% of the total amount of colloidal silicon dioxide, preferably part of the prescription The amount of croscarmellose sodium is 40~60% of the croscarmellose sodium total amount; step S2, prepares the aqueous solution of hypromellose; step S3, mixes the aqueous solution of 40~50 ℃ with The first mixture is mixed and granulated to obtain wet granules; Step S4, the wet granules are dried to obtain the first dry granules; Step S5, the remaining prescription amount of colloidal si...
Embodiment 6
[0068] Microcrystalline cellulose 102 was used to replace the microcrystalline cellulose in Example 1, and the rest were the same as in Example 1.
Embodiment 7
[0070] Adopt hypromellose E6 to replace the hypromellose E5 of embodiment 1, all the other are the same as embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap