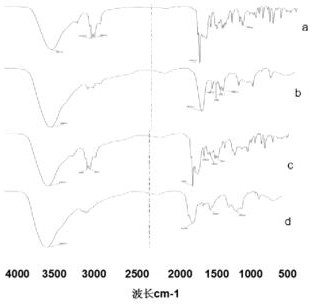
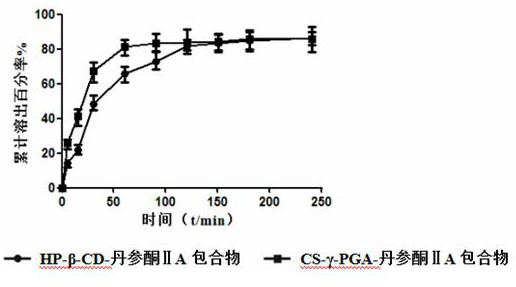
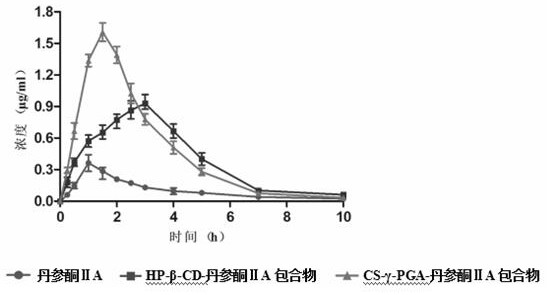
Preparation method and application of a water-soluble chitosan/γ-polyglutamic acid nanocomposite loaded with tanshinone Ⅱa
A technology of water-soluble chitosan and nano-composite, which is applied in the field of pharmaceutical and pharmacy research, can solve the problems of large administration volume, large amount of auxiliary materials, low drug loading amount, etc., and achieves improved curative effect, simple preparation method, and improved in vivo improvement. The effect of absorption properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Precisely weigh γ-PGA dissolved in ultrapure water to make 0.125 mg / ml γ-PGA aqueous solution, weigh 60mg CS and dissolve it in 10ml ultrapure water, weigh 12mg Tanshinone II A Dissolve in 12ml of absolute ethanol, Tanshinone Ⅱ A Add 12ml of γ-PGA solution with a concentration of 0.125 mg / ml to the ethanol solution, place it on a magnetic stirrer and stir for 10min at a speed of 500rpm / min, then slowly add the mixed solution dropwise to the above CS solution, and continue magnetically stirring for 1h , The speed is 1000rpm / min. After the reaction is completed, the suspension is rotary evaporated until the organic solvent is evaporated to obtain CS-γ-PGA-Tanshinone II A clathrate solution. Jiang CS-γ-PGA-Tanshinone Ⅱ A CS-γ-PGA-tanshinone Ⅱ obtained by freeze-drying the clathrate solution A Clathrate powder.
Embodiment 2
[0033] Precisely weigh γ-PGA dissolved in ultrapure water to make 0.25mg / ml γ-PGA aqueous solution, weigh 40mg CS and dissolve in 10ml ultrapure water, weigh 5mg Tanshinone II A Dissolve in 12ml of absolute ethanol, Tanshinone Ⅱ AAdd 8 ml of γ-PGA solution with a concentration of 0.25 mg / ml to the ethanol solution, place it on a magnetic stirrer and stir for 7 minutes at a speed of 300 rpm / min, then slowly add the mixed solution dropwise to the above CS solution, and continue magnetic stirring for 1.5 h, the speed is 2000rpm / min. After the reaction is completed, the suspension is rotary evaporated until the organic solvent is evaporated to obtain CS-γ-PGA-Tanshinone II A clathrate solution. Jiang CS-γ-PGA-Tanshinone Ⅱ A CS-γ-PGA-tanshinone Ⅱ obtained by freeze-drying the clathrate solution A Clathrate powder.
Embodiment 3
[0035] Precisely weigh γ-PGA dissolved in ultrapure water to make 0.4mg / ml γ-PGA aqueous solution, weigh 80mg CS and dissolve in 10ml ultrapure water, weigh 16mg Tanshinone II A Dissolve in 10ml of absolute ethanol, Tanshinone Ⅱ A Add 10ml of γ-PGA solution with a concentration of 1.6 mg / ml to the ethanol solution, place it on a magnetic stirrer and stir for 20min at a speed of 400rpm / min, then slowly add the mixed solution dropwise to the above CS solution, and continue magnetic stirring for 1h , The speed is 3000rpm / min. After the reaction is completed, the suspension is rotary evaporated until the organic solvent is evaporated to obtain CS-γ-PGA-Tanshinone II A clathrate solution. Jiang CS-γ-PGA-Tanshinone Ⅱ A CS-γ-PGA-tanshinone Ⅱ obtained by freeze-drying the clathrate solution A Clathrate powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| degree of carboxylation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com