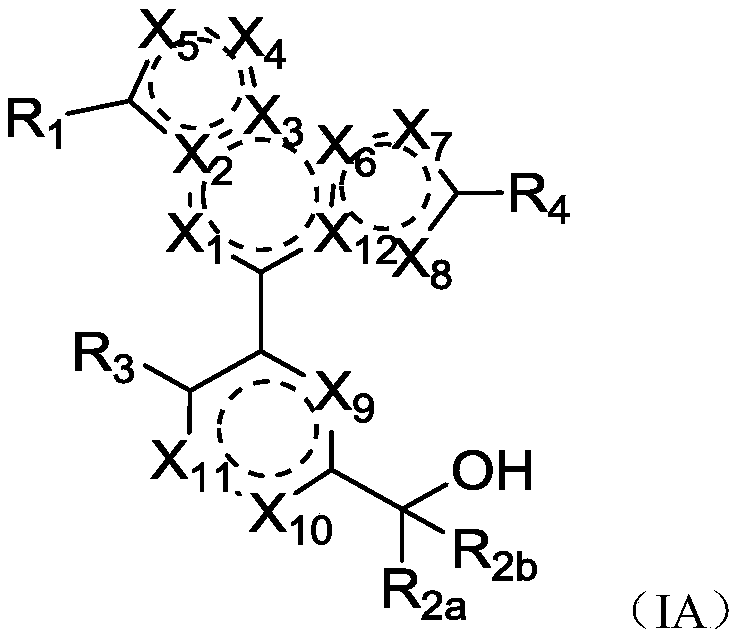
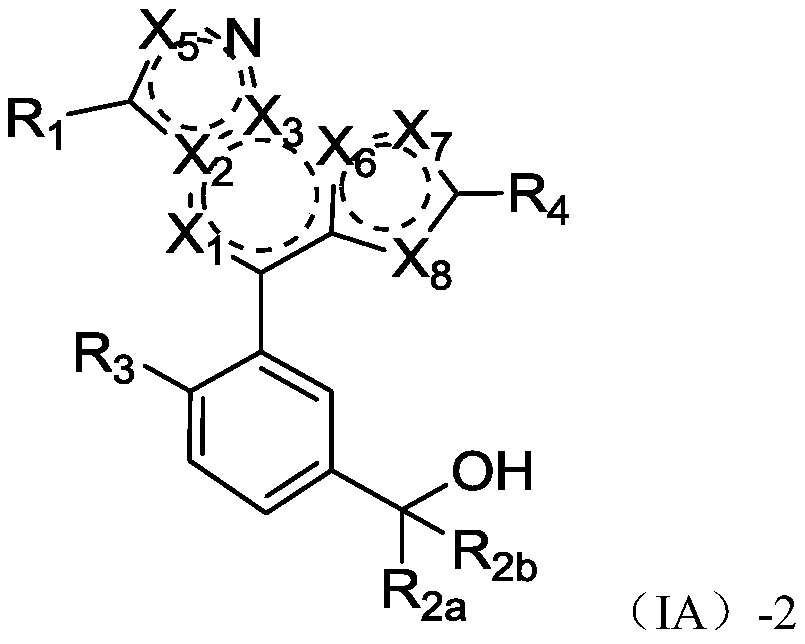
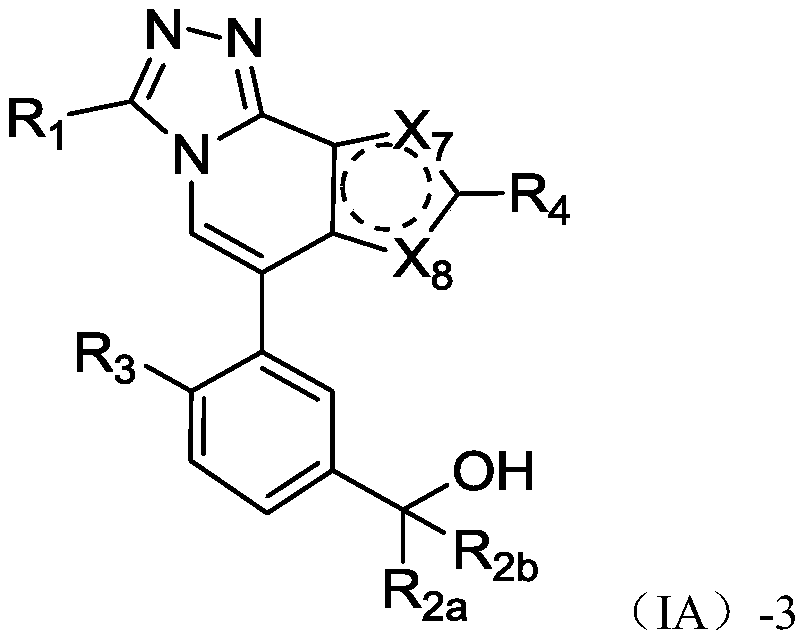
Fused heterocyclic biaryl benzyl alcohol compound, and preparation method and application thereof
A technology of aryl benzyl alcohol and heteroaryl, applied in the field of medicinal chemistry, can solve the problems of insufficient selectivity, limiting the anti-tumor potential of biological functions of bromodomain-containing proteins, and the mechanism to be further elucidated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0328] Example 1: N-ethyl-6-(2-(4-fluoro-2,6-dimethylphenoxy)-5-(2-hydroxypropyl-2-yl)phenyl)-3- Methyl-9H-pyrrole[2,3-c][1,2,4]triazol[4,3-a]pyridine-8-amide
[0329]
[0330] The first step: 4-bromo-7-chloro-1H-pyrrolo[2,3-c]pyridine-2-carboxylic acid (190mg, 0.7mmol), ethylamine hydrochloride (254mg, 3.12mmol) and HATU (593mg, 1.56mmol) was dissolved in N,N-dimethylformamide DMF (20mL), added diisopropylethylamine (DIEA) (536mg, 4.16mmol), reacted at room temperature for 3 hours, LCMS detection showed that the reaction was complete . Diluted with ethyl acetate, washed with saturated brine, dried, concentrated under reduced pressure, and purified by silica gel column chromatography to obtain 4-bromo-7-chloro-N-ethyl-1H-pyrrolo[2,3-c]pyridine-2- Amide (130 mg, yellow solid). LC-MS (M+H) 301.7 / 303.7. 1 H-NMR (400MHz, DMSO-d6) δ12.82(s, 1H), 8.50(s, 1H), 8.13(s, 1H), 7.26(s, 1H), 3.35(q, J=7.2Hz, 2H ), 1.17 (t, J=7.2Hz, 3H).
[0331] The second step: 4-bromo-7-chloro-N...
Embodiment 2
[0333] Example 2: N-ethyl-6-(2-(4-fluoro-2,6-dimethylphenoxy)-5-(2-hydroxypropyl-2-yl)phenyl)-3- Methyl-7H-pyrrole[3,2-c][1,2,4]triazol[4,3-a]pyridine-8-amide
[0334]
[0335] The first step: the compound 7-bromo-4-hydroxyl-1H-pyrrolo[3,2-c]pyridine-2-carboxylic acid (623mg, 2.4mmol) was dissolved in DMF (10mL), and HATU (1.4g, 3.6mmol) and DIEA (619mg, 4.8mmol), stirred at room temperature for 5 minutes, then added ethylamine (2M in THF, 2.4mL, 4.8mmol), and reacted overnight at room temperature. LCMS detection showed that the reaction was complete, diluted with ethyl acetate, washed with saturated brine, dried, concentrated under reduced pressure, and purified by silica gel column chromatography to obtain 7-bromo-N-ethyl-4-hydroxy-1H-pyrrolo[3,2- c] Pyridine-2-amide (695 mg, white solid). LC-MS (M+H) 284.0 / 286.0.
[0336] Second step: Dissolve 7-bromo-N-ethyl-4-hydroxy-1H-pyrrolo[3,2-c]pyridine-2-amide (695 mg, 2.4 mmol) in phosphorus oxychloride POCl 3 (6mL), heated...
Embodiment 3
[0339] Example 3: N-ethyl-6-(5-(2-hydroxypropyl-2-yl)-2-((4-methylcyclohexyl)oxo)phenyl)-3-methyl-9H -Pyrrole[2,3-c][1,2,4]triazol[4,3-a]pyridine-8-amide
[0340] The operation is the same as in Example 1, 1H-NMR (400MHz, CD3OD) δ7.96(s, 1H), 7.70(d, J=2.4Hz, 1H), 6.49(d, J=8.4Hz, 1H), 4.32–4.25 (m,1H),3.41(q,J=7.2Hz,2H),2.79(s,3H),2.12-2.06(m,2H),1.80-1.74(m,2H),1.56(s,6H), 1.53-1.47(m,2H),1.43-1.39(m,1H),1.25(t,J=7.2Hz,3H),1.07-0.95(m,2H),0.86(d,J=6.4Hz,3H) ; LC-MS (M+H) 490.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com