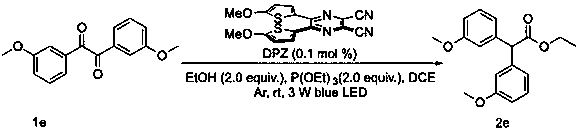
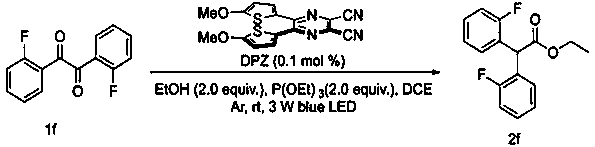
Diaryl acetate compound and preparation method thereof
A technology for diaryl acetates and compounds, which is applied in the field of compounding and preparation of diaryl acetates, can solve the problems of harsh reaction conditions and low efficiency, and achieve the effect of mild reaction conditions and high practicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0028] Example 1
[0029] Preparation of ethyl 2,2-diphenylacetate (2a):
[0030]
[0031] Specific synthesis steps and characterization:
[0032] In a 10 ml Schlenk tube, combine benzil 1a (21 mg, 0.1 mmol, 1.0 equiv.) and P(OEt) 3 (33.2mg, 0.2 mmol, 2.0 equiv.) dissolved in DCE (1.0 ml), add EtOH (11.7 μL, 0.2 mmol, 2.0 equiv.) and photocatalyst DPZ (0.0354 mg, 0.1 μmol), 3W LED at room temperature (450 nm) irradiate, stir and react for 48 hours, TLC detects that the reaction is complete. The reaction solution was concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (volume ratio, the same below, PE: EA = 20: 1) to obtain diaryl acetate compound 2a, 21.5 mg, 90%.
[0033] 1 H NMR (300 MHz, Chloroform- d ) δ 7.41 – 7.23 (m, 10H), 5.04 (s, 1H), 4.24 (q, J = 7.1 Hz, 2H), 1.28 (t, J = 7.1 Hz, 3H).
[0034] 13 C NMR (75 MHz, CDCl 3 ) δ 172.44, 138.80, 128.59, 128.54, 127.18, 61.15, 57.16, 14.12; HRMS (ESI) m / z 241.1226 (M+H + ), calc. f...
Example Embodiment
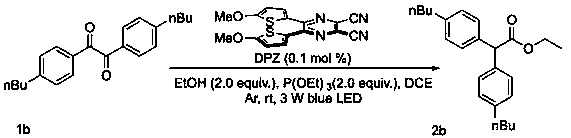
[0037] Example 2
[0038] Preparation of 2,2-bis(4-butylphenyl)ethyl acetate (2b):
[0039]
[0040] Specific synthesis steps and characterization:
[0041] In a 10 ml Schlenk tube, combine the benzyl compound 1b (32.2 mg, 0.1 mmol, 1.0 equiv.) and P(OEt) 3 (33.2 mg, 0.2 mmol, 2.0 equiv.) dissolved in DCE (1.0 ml), add EtOH (11.7 μL, 0.2 mmol, 2.0 equiv.) and photocatalyst DPZ (0.0354 mg, 0.1 μmol), 3W LED at room temperature (450 nm) irradiate, stir and react for 48 hours, TLC detects that the reaction is complete. The reaction solution was concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (volume ratio, the same below, PE: EA = 20: 1) to obtain diaryl acetate compound 2b, 27 mg, 77%.
[0042] 1 H NMR (300 MHz, Chloroform- d ) δ 7.14 (d, J = 7.9 Hz, 4H), 7.04 (d, J = 8.0 Hz, 4H), 4.86 (s, 1H), 4.11 (q, J = 7.1 Hz, 2H), 2.49 (t, J = 7.7 Hz,4H), 1.55 – 1.43 (m, 4H), 1.33 – 1.22 (m, 4H), 1.17 (t, J = 7.1 Hz, 3H), 0.83(t, J = 7.3...
Example Embodiment
[0044] Example 3
[0045] Preparation of 2,2-bis(4-chlorophenyl)ethyl acetate (2c):
[0046]
[0047] Specific synthesis steps and characterization:
[0048] In a 10 ml Schlenk tube, combine the benzil compound 1c (27.9 mg, 0.1 mmol, 1.0 equiv.) and P(OEt) 3 (33.2 mg, 0.2 mmol, 2.0 equiv.) dissolved in DCE (1.0 ml), add EtOH (11.7 μL, 0.2 mmol, 2.0 equiv.) and photocatalyst DPZ (0.0354 mg, 0.1 μmol), 3W LED at room temperature (450 nm) irradiate, stir and react for 48 hours, TLC detects that the reaction is complete. The reaction solution was concentrated under reduced pressure, and the residue was separated by silica gel column chromatography (volume ratio, the same below, PE: EA = 20: 1) to obtain the diaryl acetate compound 2c, 22 mg, 71%.
[0049] 1 H NMR (300 MHz, Chloroform- d ) δ 7.30 (d, J = 8.6 Hz, 4H), 7.22 (d, J = 8.6 Hz, 4H), 4.94 (s, 1H), 4.21 (q, J = 7.1 Hz, 2H), 1.26 (t, J = 7.1 Hz, 3H).
[0050] 13 C NMR (75 MHz, CDCl 3 ) δ 171.8, 136.8, 133.4, 129.9, 128.8, 61.5, 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com