Combined therapy for n-methyl-d-aspartic acid receptor antagonist-responsive neuropsychiatric disorders
A technology for neuropsychiatric diseases and glutamate receptors, applied in neurological diseases, active ingredients of hydroxyl compounds, drug combinations, etc., can solve problems such as increasing the risk of side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
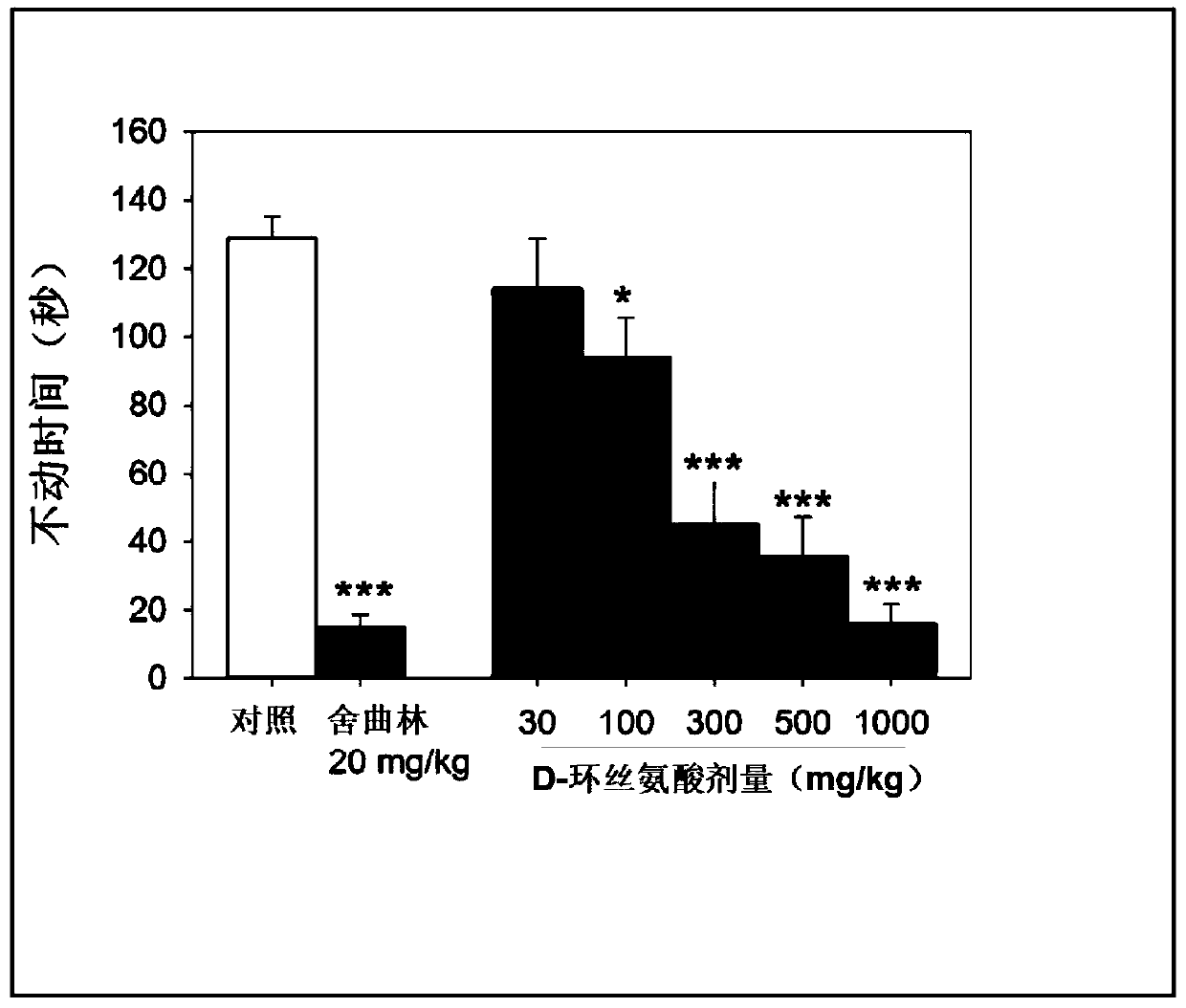
[0137] Example 1: Demonstration of the hyperkinetic and antidepressant effects of D-cycloserine on rodents
[0138] For the present study, the psychomotor effects of D-cycloserine were assessed using rodent open field tests following D-cycloserine administration in the presence or absence of antidepressants.
[0139] All tests were performed at PsychoGenics Inc, 765 Old Saw Mill River Road, Tarrytown, NY 10591, USA.
[0140] Male C57BL / 6J mice (8 weeks old) from Jackson Laboratories (Bar Harbor, Maine) were used. Upon receipt, the mice were assigned a unique identification number (tail) and housed in OPT mouse cages in groups. All animals were acclimatized to the new colony room for 1 week prior to testing. During the acclimatization period, animals were regularly checked, handled and weighed to ensure adequate health and fitness. Animals were maintained on a 12 / 12 light / dark cycle. The room temperature is kept between 20 and 23°C, and the relative humidity is kept between...
Embodiment 2
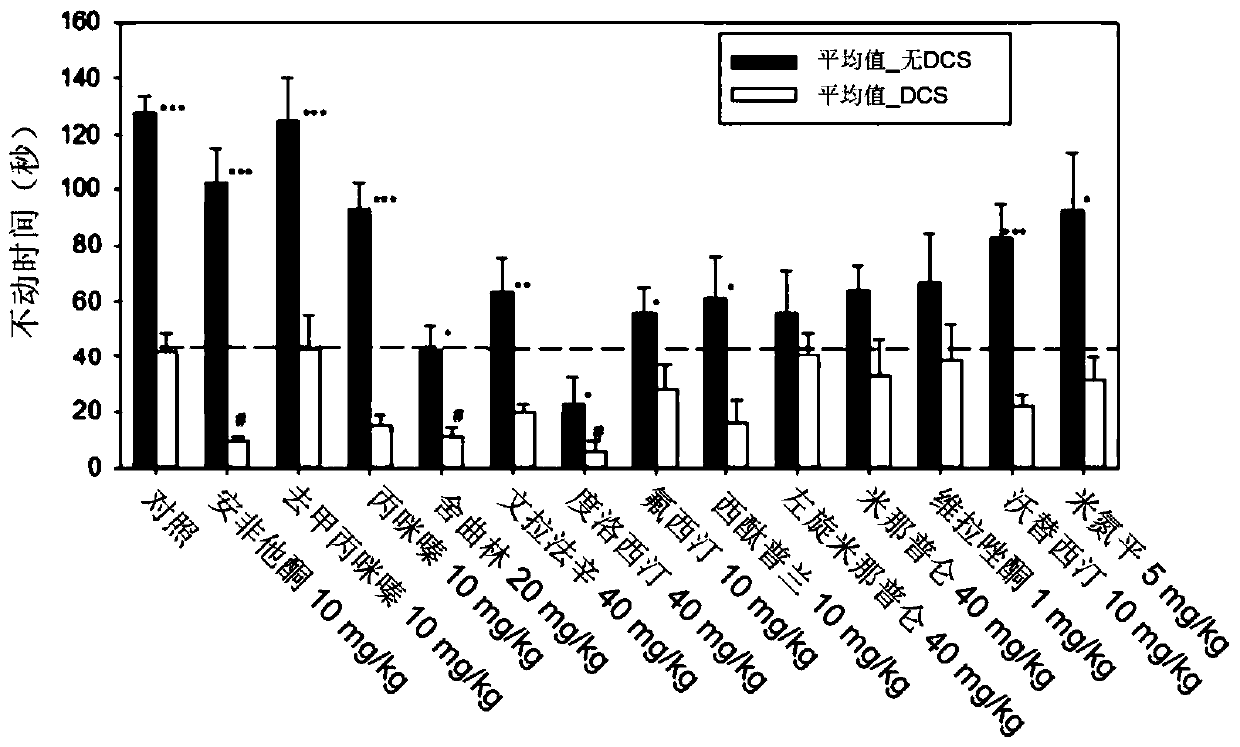
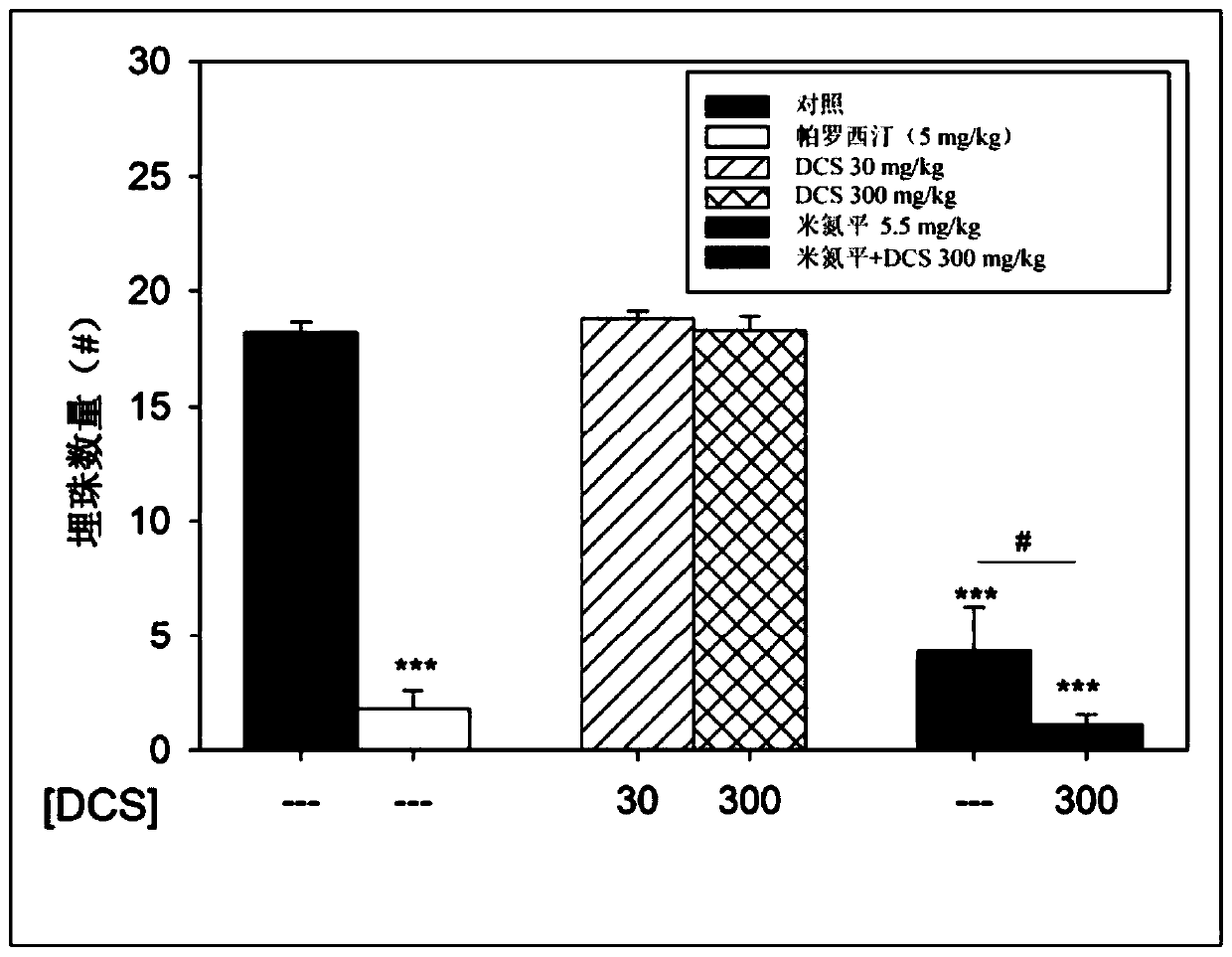
[0167] Example 2: NMDAR Antagonists Alone and in Combination with Antidepressants and Effect of Antidepressants in the Rodent Forced Swim Test
[0168] For the present study, the antidepressant effects of NMDAR antagonists were assessed using the rodent forced swim test. NMDAR antagonists alone and in combination with specific antidepressants have been studied.
[0169] All tests were performed at PsychoGenics Inc, 765 Old Saw Mill River Road, Tarrytown, NY 10591, USA.
[0170] Male C57BL / 6J mice (8 weeks old) from Jackson Laboratories, Bar Harbor, ME were used. Upon receipt, the mice were assigned a unique identification number (tail) and housed in OPT mouse cages in groups. All animals were acclimatized to the new colony room for 1 week prior to testing. During the acclimatization period, animals were regularly checked, handled and weighed to ensure adequate health and fitness. Animals were maintained on a 12 / 12 light / dark cycle. The room temperature is kept between 20 ...
Embodiment 3
[0191] Example 3: Pharmacokinetics of DCS in Rodents
[0192]In the present study, the pharmacokinetics of D-cycloserine in rodents was evaluated. This study tested the hypothesis that the antidepressant effects of DCS in the above examples were specifically observed at therapeutic levels that produced sustained blood DCS levels >25 micrograms / mL.
[0193] In this study, male C57BL / 6J mice (8 weeks old) from Jackson Laboratories in Bar Harbor, Maine were used. DCS (30, 100, 300, 500 and 1000 mg / kg) was dissolved in PTS solvent (5% PEG200: 5% Tween 80: 90% NaCl) and administered by intraperitoneal injection at a dose of 10 mL / kg.
[0194] For each treatment group, a total of 12 mice were dosed: 4 mice were collected at 30, 60 and 120 minutes. Average plasma levels are calculated within 30-60 minutes.
[0195] Analysis of DCS in plasma was accomplished using a UPLC / MS / MS system consisting of an AcquityUPLC chromatography system from Waters and a Quattro Premier XE triple quad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com