Method for preparing optically active citronellal, and catalyst used in method
An optically active, citronellal technology, used in physical/chemical process catalysts, organic chemistry methods, chemical instruments and methods, etc., can solve problems such as complex process operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
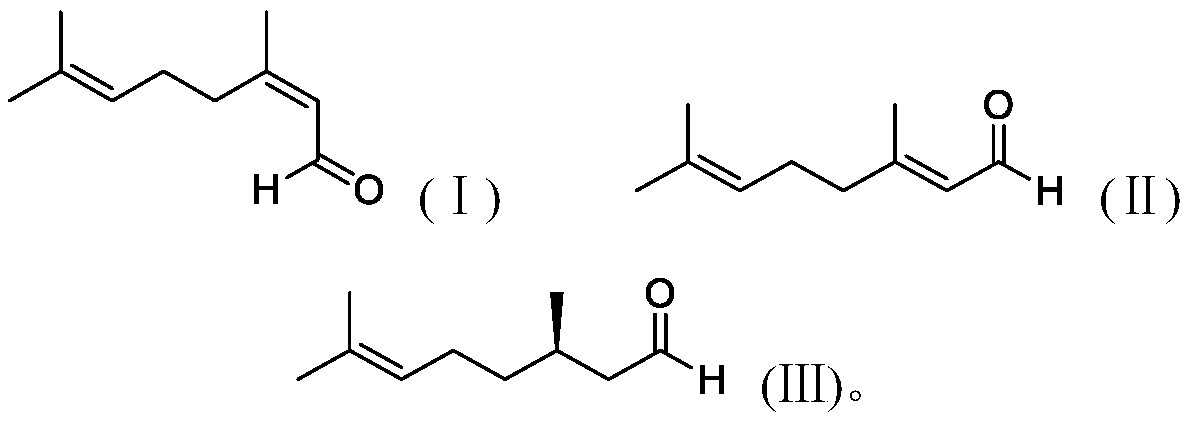
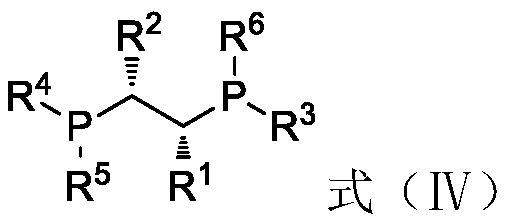
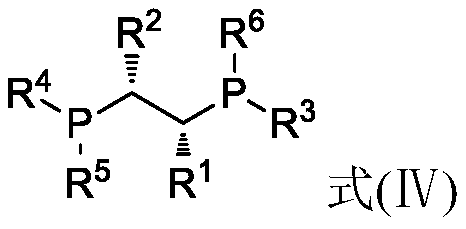
[0058] Under nitrogen atmosphere, 7.5mg Rh(CO) 2 acac (0.029mmol) and 18.6mg (R,R)-chiraphos (0.0436mmol) were dissolved in 50g toluene to obtain a solution, and 6g of 100-200 mesh basic alumina was added to the above-obtained solution under stirring, and transferred into an autoclave purged with nitrogen. Add 600g of neral aldehyde (the ratio of neral / geranial double bond isomers=99:1; based on the rhodium atom in the catalyst, the ratio of substrate / catalyst=135789) into the reactor, at 25°C After stirring for 2 hours, the reaction pressure was adjusted to 5MPa by injecting hydrogen, and the temperature was raised to 60° C. for 5 hours. The yield was 95% as measured by gas chromatography, and the yield of R-citronellal with an optical purity of 88ee% was 94%.
[0059] Based on R-citronellal and Rh(CO) 2 The turnover number for the entire reaction of acac is 127642.
Embodiment 2-4
[0061] Under nitrogen atmosphere, 7.5mg Rh(CO) 2 acac (0.029mmol) and 6.5mg (S,S)-chiraphos (0.0152mmol) were dissolved in 50g toluene to obtain a solution, and 0.2g of basic alumina was added to the solution obtained above under stirring, and it was transferred to the in a replaced autoclave. 441.8g of geranial (the ratio of geranial / neral double bond isomers=99:1; in terms of rhodium atoms in the catalyst, substrate / catalyst ratio=100000) was added to the reactor, at 25 After stirring for 2 hours at °C, the reaction pressure was adjusted to 8 MPa by injecting hydrogen, and the temperature was raised to 30 °C for 24 hours. The yield and optical purity were listed in Table 1 by gas chromatography.
[0062] After distilling off the toluene and the product, 441.8g of geranial was added to the reaction kettle, the reaction pressure was adjusted to 8MPa by injecting hydrogen, and the temperature was raised to 90°C for 8 hours. The yield and optical purity were measured by gas chr...
Embodiment 5-8
[0067] Embodiment 5-8 (preparation R-citronellal)
[0068] Under nitrogen atmosphere, 0.015mmol transition metal compound (see "transition metal compound" in Table 2 below) and 0.015mmol chiral bidentate bisphosphine ligand (see "ligand" in Table 2 below) and 100-200 Add 600g of neral aldehyde to 6 g of mesh basic alumina (the molar ratio of neral / geranial double bond isomers=99:1; the molar ratio of substrate / catalyst (based on the transition metal in the catalyst)=263157) , transferred it to an autoclave replaced with nitrogen, stirred at 25°C for 2 hours, and then adjusted a certain pressure by injecting hydrogen (see "Pressure" in Table 2 below for details), and raised the temperature to a certain temperature (see Table 2 for details) 2 "temperature"), after the reaction, the yield of R-citronellal was measured by gas chromatography, and the turnover number of the entire reaction was calculated based on the molar amount of R-citronellal and the molar amount of the transiti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com