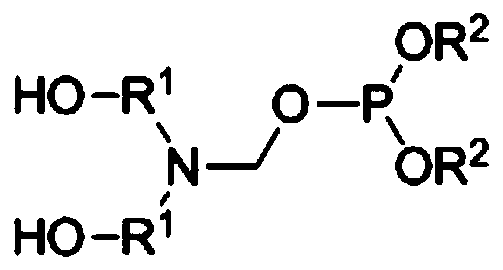
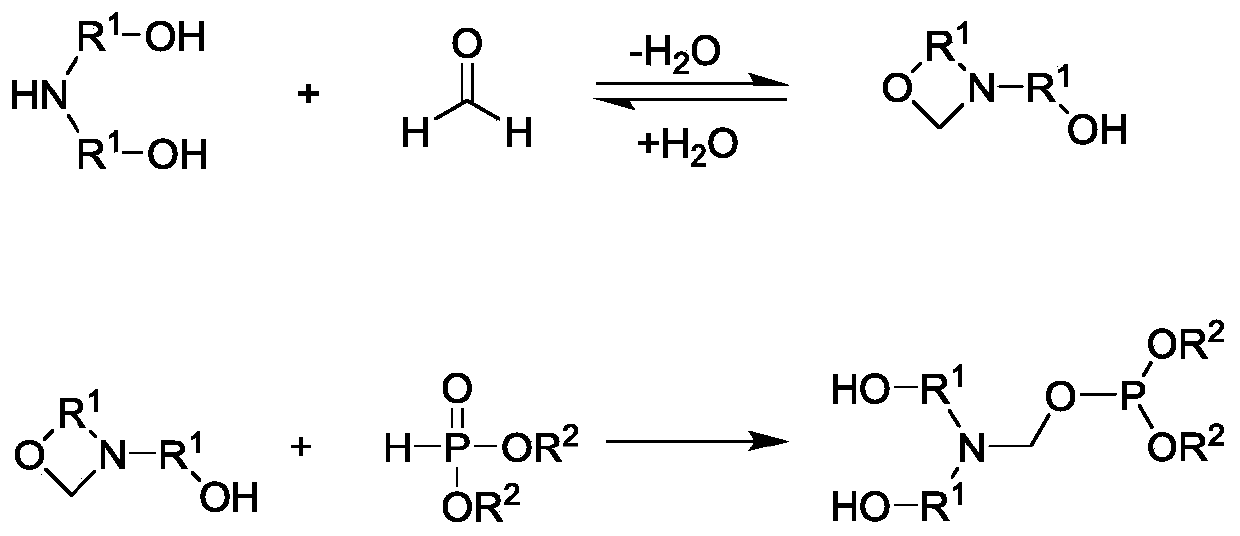
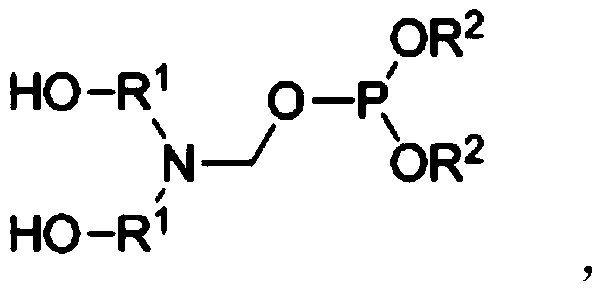
Preparation method of reactive halogen-free nitrogen-phosphorus flame retardant
A nitrogen-phosphorus flame retardant and reactive technology, applied in the field of flame retardant materials, can solve the problems of shrinkage and closed cells of polyurethane foam products, high product acid value, unfavorable polyurethane foaming reaction, etc. Avoid the effect of intermediate decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] (1) Calculate by molar mass, take by weighing paraformaldehyde: alcohol amine=1:1, be converted into mass parts as, 30 parts of paraformaldehyde and 105 parts of diethanolamine, the paraformaldehyde that is ground into powder will join in Stir in a flask containing diethanolamine, control the temperature at 40°C, and continue the reaction for 1 hour after the paraformaldehyde is completely dissolved to obtain the intermediate 3-(2-hydroxyethyl)-1,3-oxazolane solution.
[0043] (2) The intermediate 3-(2-hydroxyethyl)-1,3-oxazolane solution obtained in step (1) was decompressed with an oil pump at 80°C (gauge pressure -0.097Mpa) After dehydration for 1 h, the intermediate 3-(2-hydroxyethyl)-1,3-oxazolane was obtained.
[0044] (3) Control the temperature of the intermediate 3-(2-hydroxyethyl)-1,3-oxazolane obtained in step (2) at 60°C, and add 110 parts of dimethyl phosphite dropwise while stirring , After the dropwise addition was completed, it was reacted for 2 hours ...
Embodiment 2
[0047] (1) Calculate by molar mass, take by weighing paraformaldehyde:alcohol amine=2:1, convert into mass parts as, 60 parts of paraformaldehyde and 105 parts of diethanolamine, the paraformaldehyde that will be ground into powdery joins Stir in a diethanolamine flask, control the temperature at 45°C, and continue the reaction for 1.5 hours after the paraformaldehyde is completely dissolved to obtain the intermediate 3-(2-hydroxyethyl)-1,3-oxazolane solution .
[0048] (2) The 3-(2-hydroxyethyl)-1,3-oxazolane intermediate solution obtained in step (1) was decompressed with an oil pump at 70°C (gauge pressure-0.097Mpa) After dehydration for 1 h, the intermediate 3-(2-hydroxyethyl)-1,3-oxazolane was obtained.
[0049] (3) Control the temperature of the intermediate 3-(2-hydroxyethyl)-1,3-oxazolane obtained in step (2) at 60°C, and add 138 parts of diethyl phosphite dropwise while stirring , After the dropwise addition was completed, the mixture was reacted for 2 hours to obta...
Embodiment 3
[0052] (1) Calculate by molar mass, take by weighing paraformaldehyde:alcohol amine=2:3, be converted into mass parts as, 30 parts of paraformaldehyde and 157.5 parts of diethanolamine, the paraformaldehyde that will be ground into powder is added to Stir in a diethanolamine flask, control the temperature at 50°C, and continue the reaction for 2 hours after the paraformaldehyde is completely dissolved to obtain the intermediate 3-(2-hydroxyethyl)-1,3-oxazolane solution.
[0053] (2) The intermediate 3-(2-hydroxyethyl)-1,3-oxazolane solution obtained in step (1) was decompressed with an oil pump at 90°C (gauge pressure -0.098Mpa) After dehydration for 1 h, the intermediate 3-(2-hydroxyethyl)-1,3-oxazolane was obtained.
[0054] (3) Control the temperature of the intermediate 3-(2-hydroxyethyl)-1,3-oxazolane obtained in step (2) at 70°C, and add 166 parts of diisopropyl phosphite dropwise while stirring The ester was reacted for 4 hours after the dropwise addition to obtain 292...
PUM
| Property | Measurement | Unit |
|---|---|---|
| acid value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com