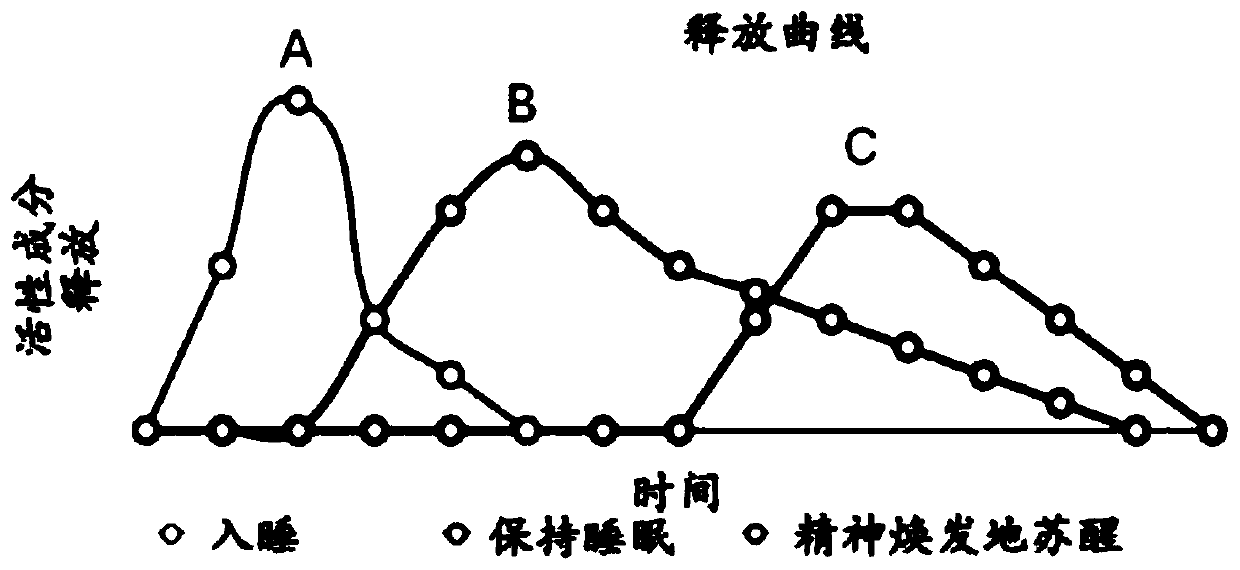
Time release sleep aid system
A delayed release, sleep technology, applied in nervous system diseases, peptide/protein components, drug combinations, etc., can solve the problems of drugs that cannot continue to work for a long time, poor effect, side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0086] Example 1: Preparation of soft chewable tablet compositions
[0087] Capsules were prepared as detailed in Table 1 and as follows. Powders from each of the following active ingredients were prepared by mixing with the polymeric binder in a low shear planetary mixer for 3 minutes:
[0088] Valerian Root Extract
[0089] PHARMAGABA TM
[0090] 5-HTP (5-Hydroxytryptophan)
[0091] melatonin, and
[0092] SUNTHEANINE TM
[0093] Water is then added to the powder mixture to create a wet mass. The wet mass was passed through the extruder at 50 rpm. The extrudate was spheronized in a spheronizer at 1000 rpm for 1 minute to produce spherical beads. The spherical beads were dried until the moisture content was reduced to less than 5 wt%.
[0094] Prepare RELORA containing RELORA as described above TM Compositions with valerian root extract, except that a taste-masking agent is used instead of a polymeric binder.
[0095] By combining valerian root extract, PHARMAGABA...
example 2
[0103] Example 2: Preparation of soft chewable tablet compositions
[0104] Capsules were prepared as detailed in Table 2 and as follows. Powders from each of the following active ingredients were prepared by mixing with the polymeric binder in a low shear planetary mixer for 3 minutes:
[0105] 5-HTP (5-Hydroxytryptophan)
[0106] melatonin, and
[0107] SUNTHEANINE TM
[0108] Water is then added to the powder mixture to create a wet mass. The wet mass was passed through the extruder at 50 rpm. The extrudate was spheronized in a spheronizer at 1000 rpm for 1 minute to produce nanobeads. The nanobeads were mixed with various excipients and water in a low shear planetary mixer for 3 minutes to create a wet mass. The wet mass was passed through the extruder at 50 rpm. The extrudate was spheronized in a spheronizer at 1000 rpm for 1 minute to produce microbeads. The microbeads were dried until the moisture content was reduced to less than 5 wt%.
[0109] Capsules are p...
example 3
[0114] Example 3: Exemplary Compositions
[0115] Tables 4 to 6 show exemplary sleep-promoting active ingredients, sleep-quality active ingredients, sleep-restoring active ingredients, and next-day active ingredients, the amount and function of each ingredient.
[0116] Table 4: Exemplary Sleep Promoting Active Ingredients and Sleep Quality Active Ingredients
[0117]
[0118]
[0119]
[0120] Table 5: Exemplary Sleep Restoring Active Ingredients
[0121]
[0122]
[0123]
[0124] Table 6: Exemplary Next-Day Active Ingredients
[0125]
[0126]
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap