Mavacamten for use in the treatment of hypertrophic cardiomyopathy
A kind of hypertrophic cardiomyopathy, general technology, applied in the treatment of myocardial diastolic dysfunction, the field of treatment of hypertrophic cardiomyopathy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0263] Example 1. Preparation of (S)-3-isopropyl-6-((1-phenylethyl)amino)pyrimidine-2,4(1H,3H)-dione.
[0264]
[0265] Compound 1.1. Isopropylurea. To isopropylamine (15.3 g, 0.258 mol, 1.0 equiv) in CH at 0 °C under argon 2 Cl 2 To a stirred solution in (200 mL) was added trimethylsilyl isocyanate (30 g, 0.26 mol, 1.0 equiv) dropwise. The resulting mixture was allowed to come to ambient temperature and stirred overnight. After cooling to 0°C, CH was added dropwise 3 OH (100 mL). The resulting solution was stirred at room temperature for 2 hours (h) and then concentrated under reduced pressure. The crude residue was converted from CH 3 OH:Et 2 Recrystallization from O (1:20) yielded 15.4 g (58%) of the title compound as a white solid. LC / MS: m / z(ES+)103(M+H) + .
[0266]
[0267] Compound 1.2.1-Isopropylbarbituric acid. To 1.1 (14.4g, 0.14mol, 1.00 equiv) in CH 3 To a stirred solution in OH (500 mL) was added dimethyl malonate (19.55 g, 0.148 mol, 1.05 eq)...
Embodiment 2I
[0272] Embodiment 2. Phase I clinical research
[0273] Three clinical studies have investigated the safety and tolerability of Compound 1 to date; the conduct of 2 studies has been completed and 1 study is ongoing. A double-blind, placebo-controlled, single ascending dose study (SAD) (Compound 1-002) was conducted in healthy men. Subjects receive a single dose ranging from 1 mg to 48 mg. An open-label sequential cohort SAD study (Compound 1-001) in clinically stable HCM was also completed; single doses up to 144 mg were administered. In addition, a double-blind, placebo-controlled, multiple ascending dose (MAD) study (Compound 1-003) is ongoing in which healthy adult subjects receive multiple doses of Compound 1 up to 25 mg once daily (QD), Lasts up to 28 days.
[0274]
[0275]
[0276] (1) 48 mg cohort (n=4 patients); 96 mg cohort (n=6); 144 mg cohort (n=5).
[0277] (2) Each cohort is randomized 6:2 to Compound 1 or matching placebo.
[0278] (3) Each cohort i...
Embodiment 3
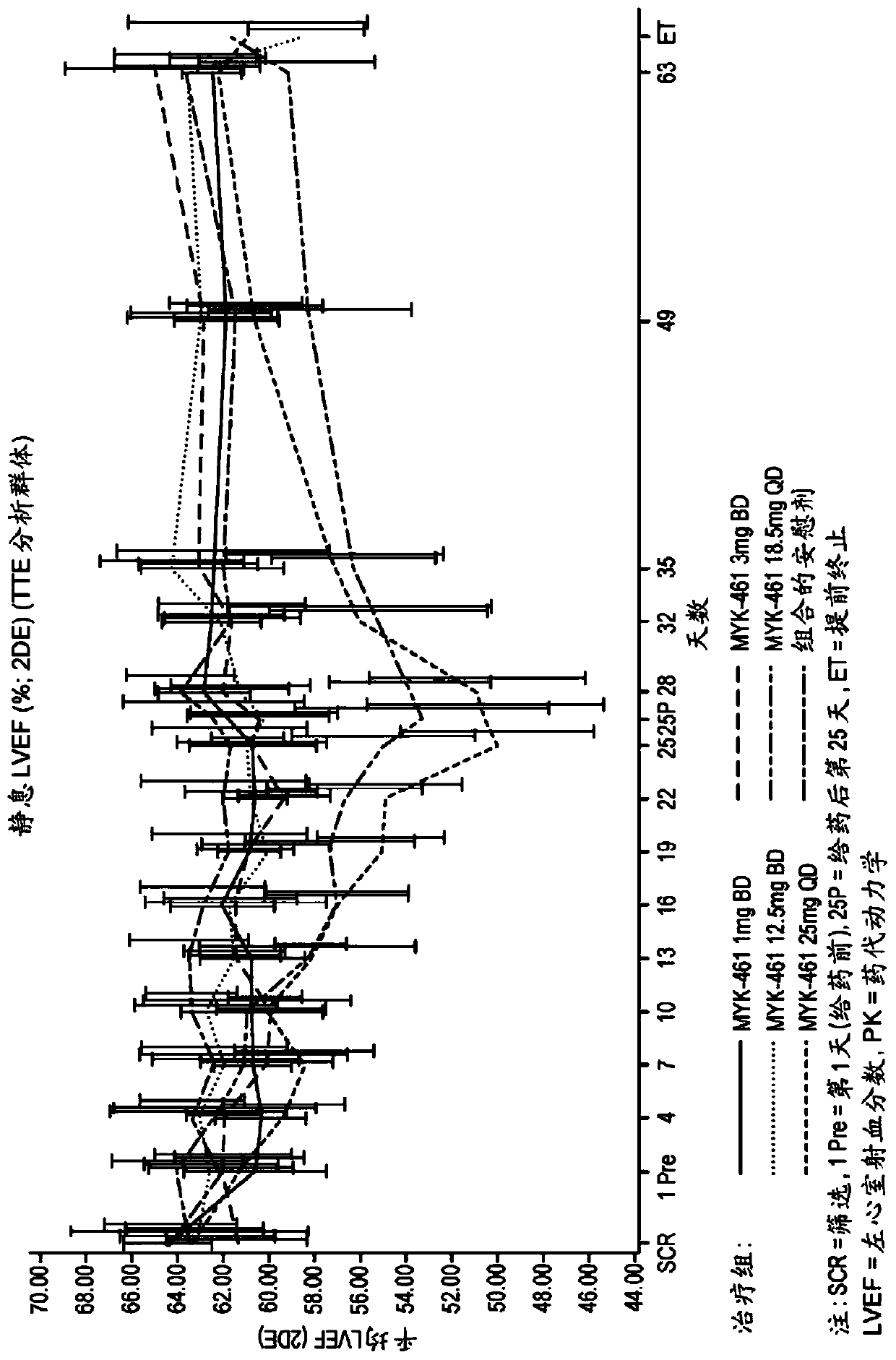
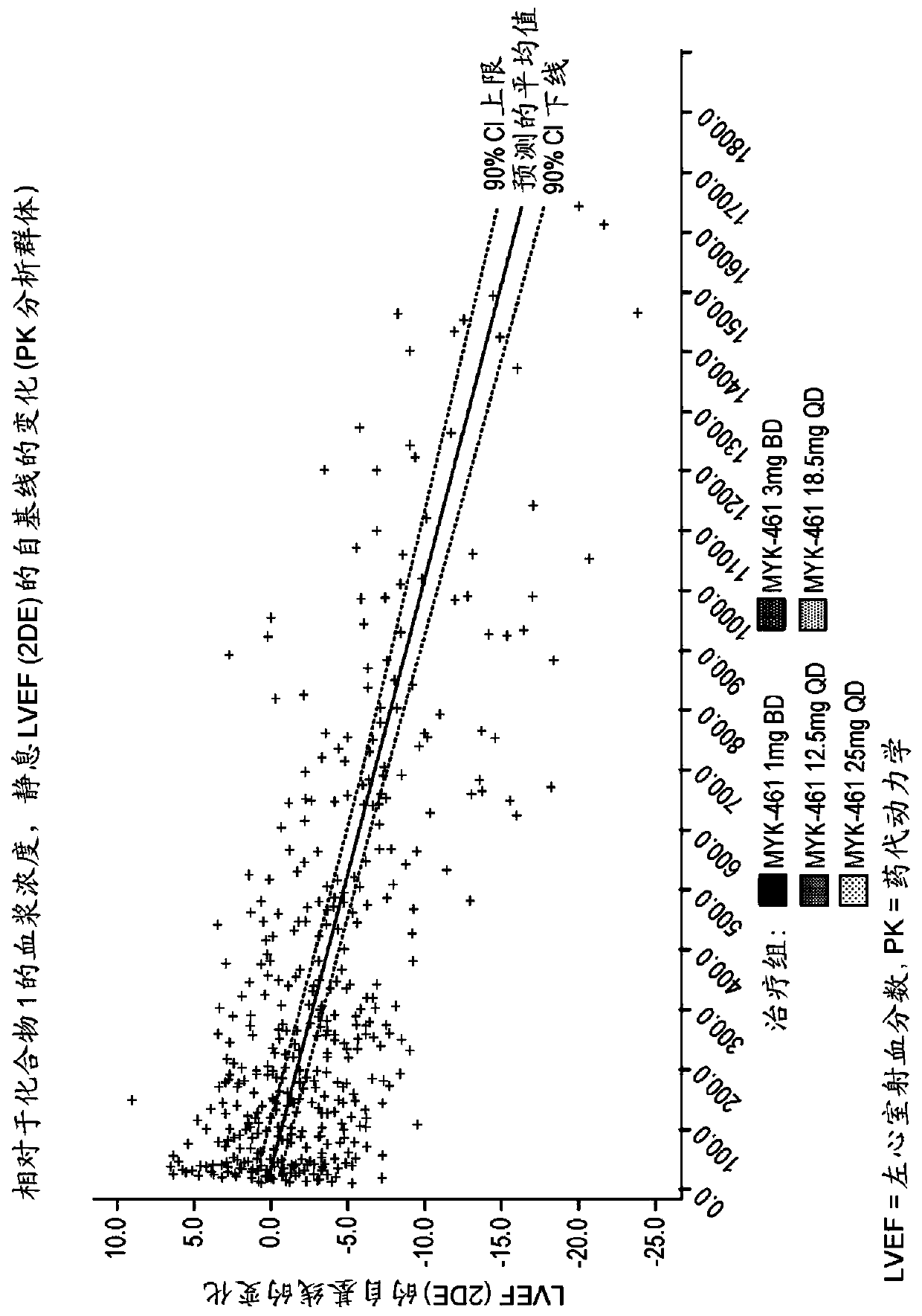
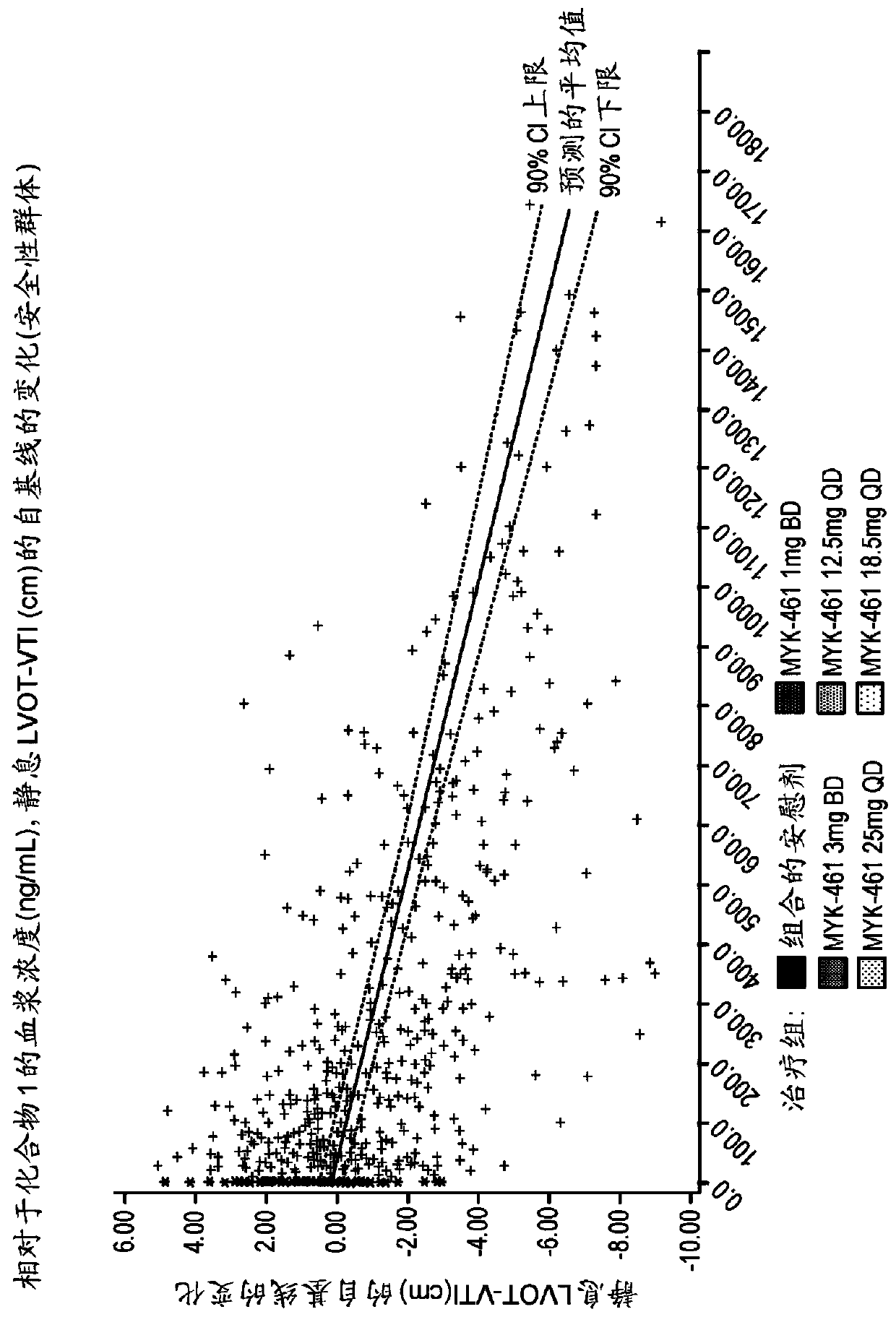
[0302] Example 3. For evaluation in subjects with symptomatic hypertrophic cardiomyopathy and left ventricular outflow tract obstruction Phase 2 Development Label Preliminary Study of Efficacy, Pharmacokinetics, Pharmacodynamics, Safety and Tolerability of Compound 1 (Pioneer-HCM-A)
[0303] Research purposes:
[0304] the main purpose:
[0305] To characterize the effect of 12 weeks of Compound 1 treatment on reducing post-exercise peak left ventricular outflow tract (LVOT) gradient in subjects with symptomatic hypertrophic cardiomyopathy (HCM) and LVOT obstruction
[0306] Secondary purpose:
[0307] Proportion of subjects achieving LVOT gradient response with peak post-exercise gradient <30 mm Hg assessed in subjects with symptomatic HCM and LVOT obstruction
[0308] Evaluation of 12 weeks of Compound 1 treatment on dyspnea symptom score, peak oxygen consumption (pVO) in subjects with symptomatic HCM and LVOT obstruction 2 ) and expiratory volume (VE) / carbon dioxid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Body mass index | aaaaa | aaaaa |
| Gradient | aaaaa | aaaaa |
| Gradient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com