Anrastone compounds, preparation method thereof, and application of compounds in preparation of anti-allergic drugs
A compound and anti-allergic technology, applied in the preparation of anti-allergic drugs, andrastone compounds and their preparation fields, can solve problems such as adverse reactions and easy drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
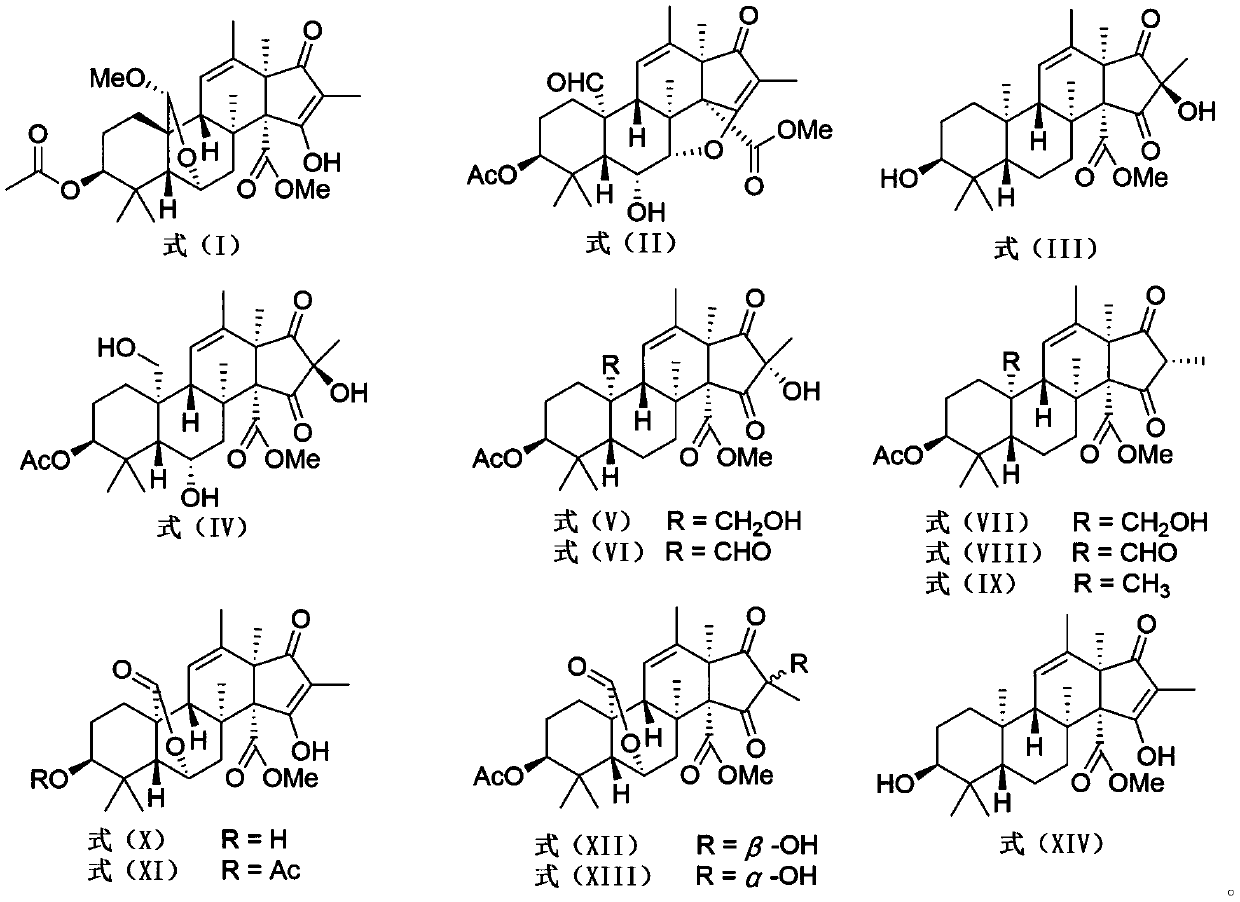
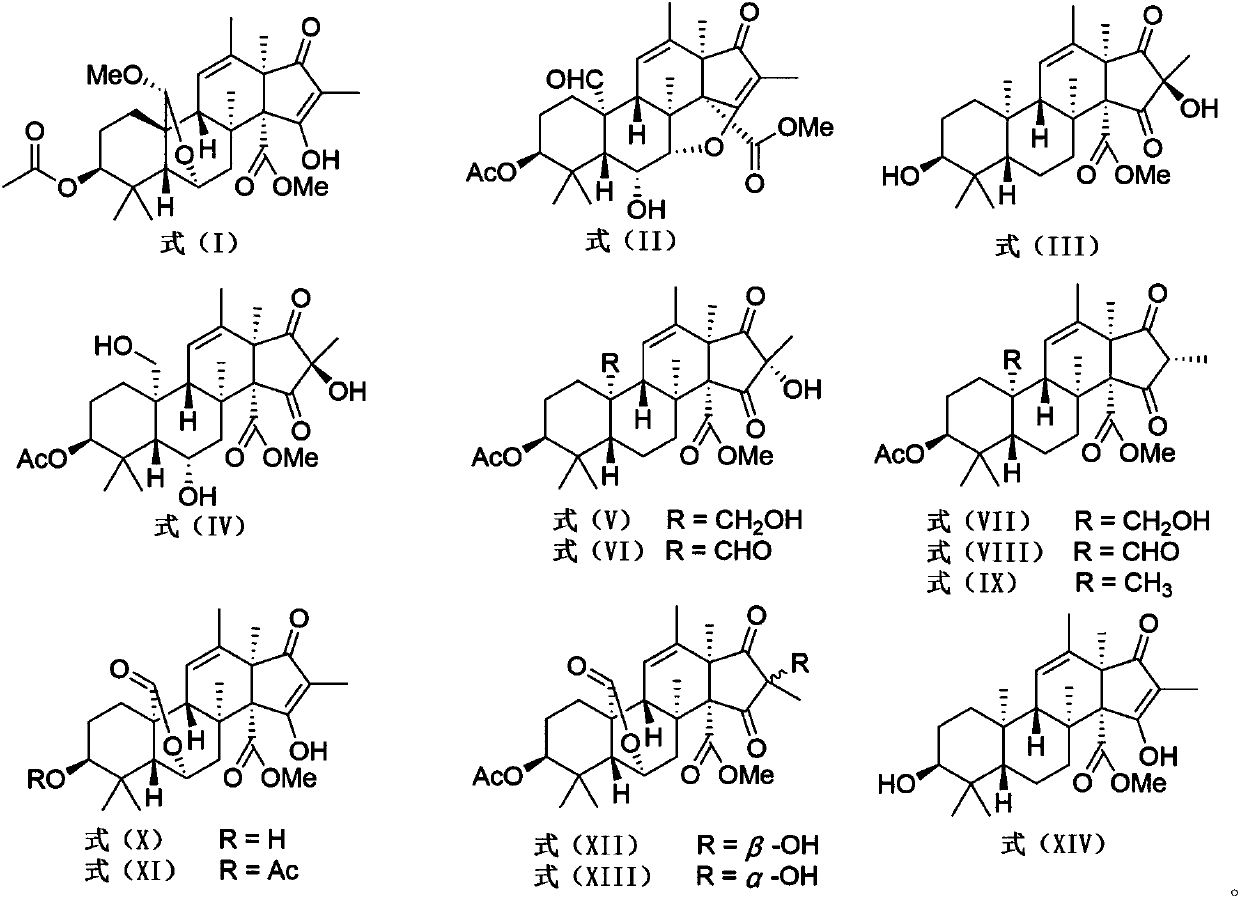
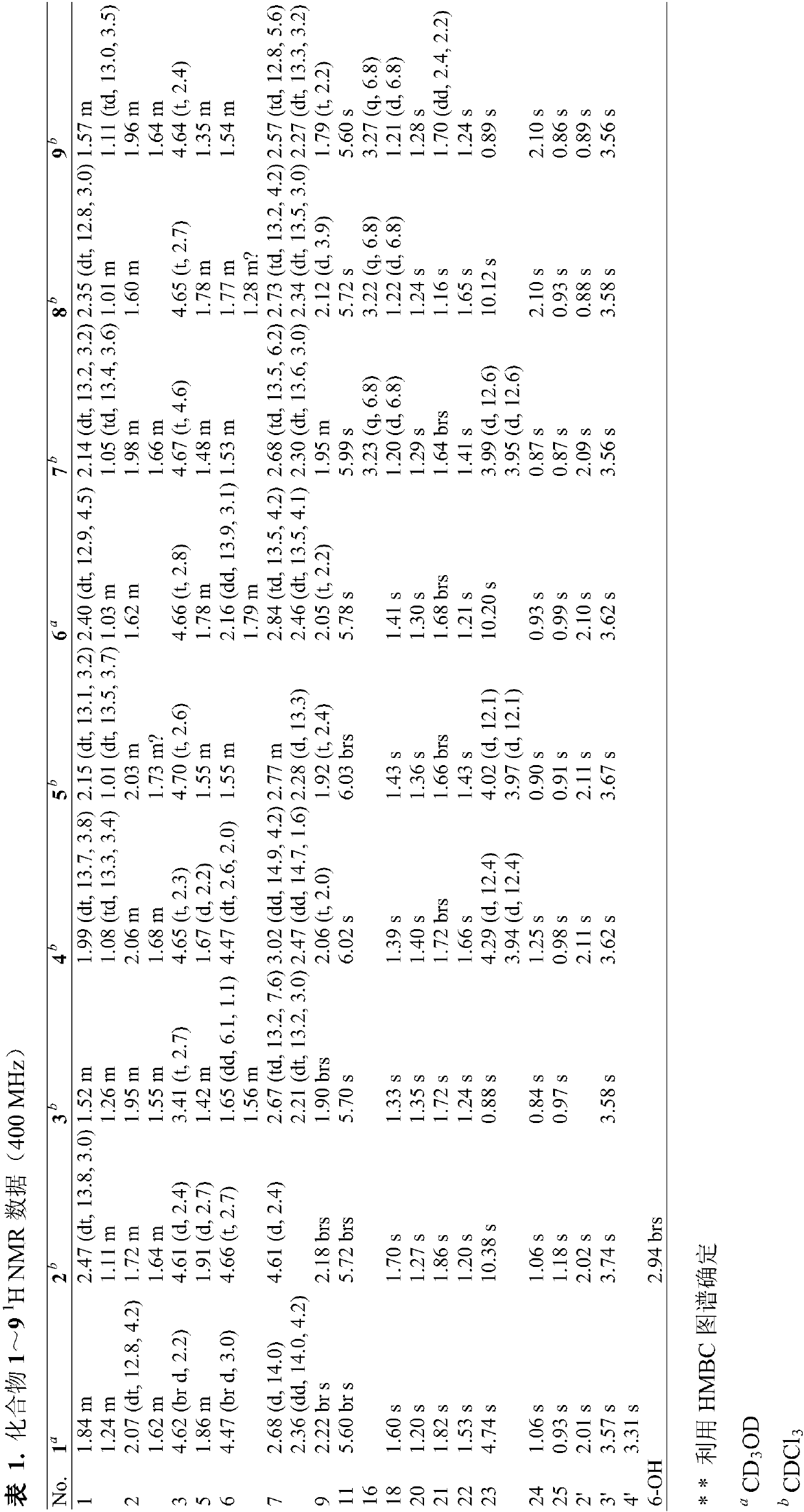
[0017] Embodiment 1: Preparation of andrastone compounds
[0018] (1) Penicillium allii-sativi was cultured on a PDA plate at 25° C. for 3 days. The fresh mycelium was then inoculated into the culture medium containing 400 mL of PDB. After 24 hours, 10 mL of seed solution was inoculated into 1 L Erlenmeyer flasks (100 bottles), and each bottle had 80 g of oats and 120 mL of 3% seawater, and cultured statically at 28° C. for 30 days. The Penicillium allii-sativi is fermented and cultivated to obtain a fermented product;
[0019] The Penicillium allii-sativi is preserved in the China Marine Microorganism Culture Collection Management Center, and the preservation number is: MCCC 3A00580.
[0020] (2) The fermented product obtained in step (1) was extracted three times with ethyl acetate. The organic solvent was evaporated under reduced pressure to obtain an organic extract (200 g). These extracts were passed through a normal-phase column chromatography and eluted with petrole...
Embodiment 2
[0034] Example 2: Investigation of the Antiallergic Activity of Compounds 1-14 Prepared in Example 1
[0035] Rat Basophilic Leukemia-cell (RBL-cell) has a good allergic immune response in the diagnosis and immunotherapy of food allergy. The RBL-2H3 cell line has high viability and good uniformity, and can be used to establish cell models of allergic reactions. Therefore, the RBL-2H3 cell model is widely used in the anti-food allergy research of natural active ingredients, including degranulation, release of allergic mediators and their mechanism of action. The RBL-2H3 cell model mediated by immunoglobulin (IgE) was used to detect the degranulation efficiency of the cells after sensitization, and the inhibition rate of the compound samples on the degranulation efficiency of the cells was calculated to judge the anti-allergic activity of the samples.
[0036] The detailed steps are as follows:
[0037] 1) RBL-2H3 cells were recovered by trypsinization, 6×10 5 cells / mL, add 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com