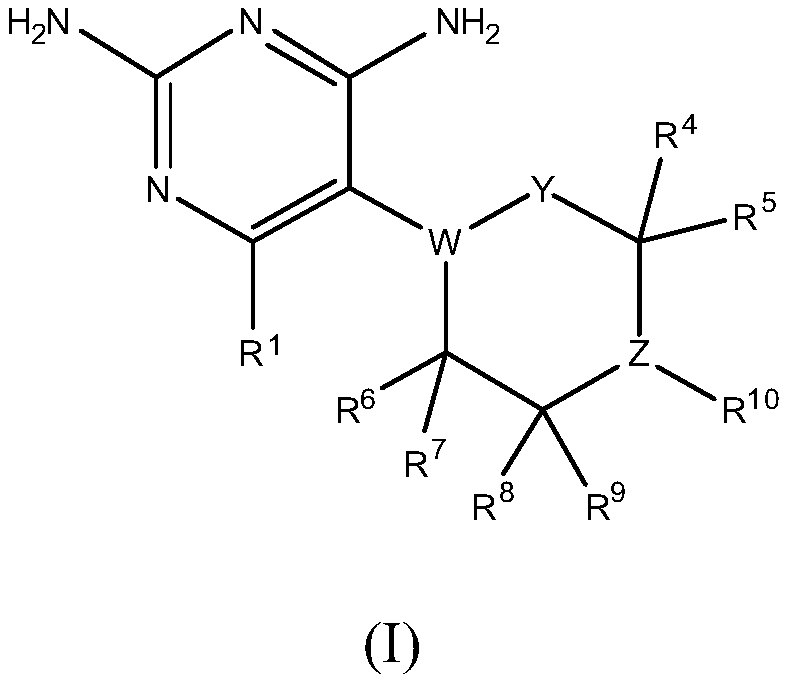
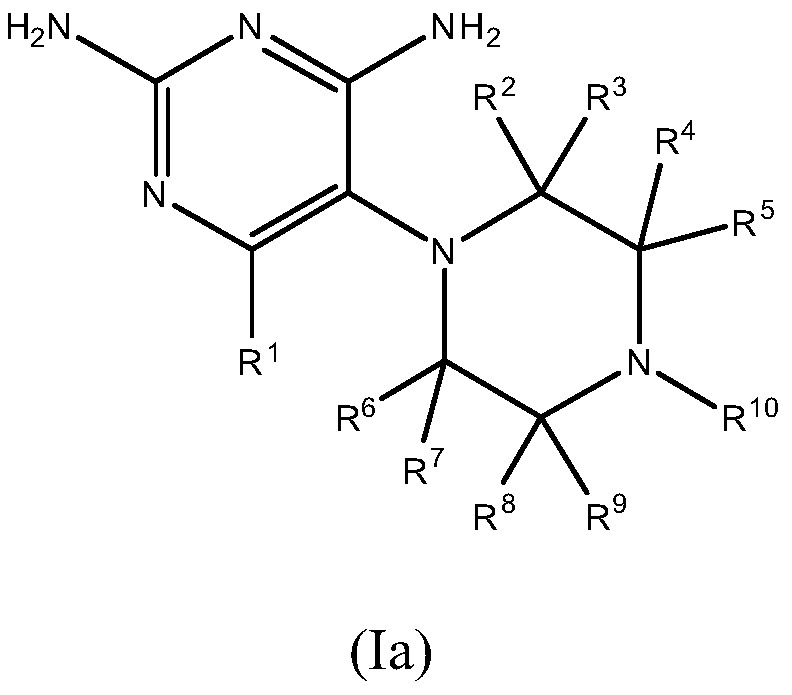
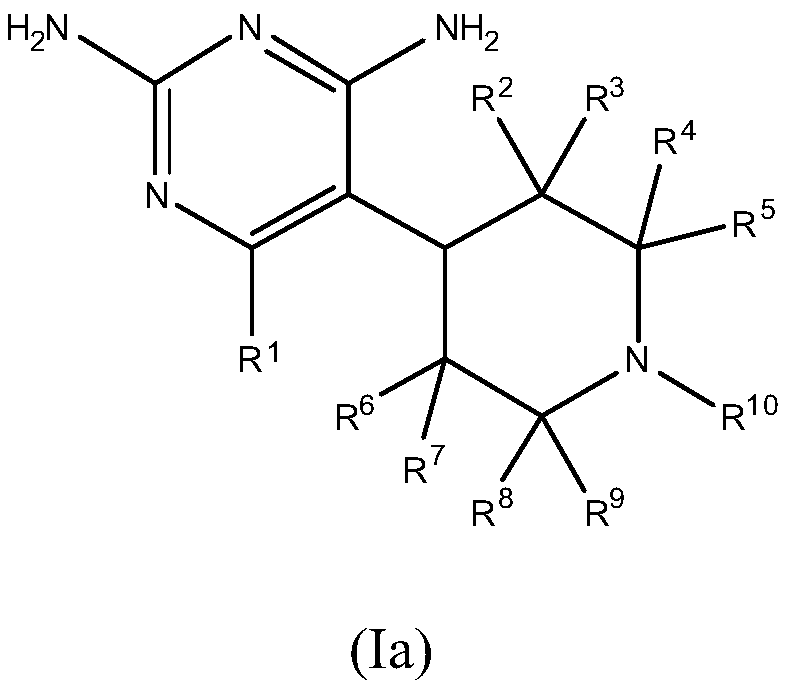
Dhfr inhibitors, compositions, and methods related thereto
A compound, R12 technology, applied in DHFR inhibitors, compositions and related fields, can solve side effects and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0238] Example 1: General method
[0239] for 1 H NMR, NMR spectra were recorded on Varian 400 MHz. Quadrupole operating in ES(+) ionization mode on Shimadzu LCMS 2010 (column: sepaxODS 50 x 2.0mm, 5um) or Agilent 1200HPLC, 1956MSD (column: Shim-pack XR-ODS 30 x 3.0mm, 2.2um) LCMS was performed on a mass spectrometer.
[0240] LC / MS method A: run at 40°C on a Shimadzu LC-20AB with MS 2010 detector using a Luna-C18(1) column (2.0*30mm, 3um). Mobile phase A was 0.037% (v / v) TFA in water and mobile phase B was 0.018% (v / v) TFA in acetonitrile. The flow rate was 0.8 mL / minute from 0.01 to 1.51 minutes and then 1.2 mL / minute from 1.52 to 2.00 minutes. The gradient was from 90% mobile phase A to 10% mobile phase A over 1.15 minutes, then held at 10% mobile phase A until 1.65 minutes, then back to 90% mobile phase A at 1.66 minutes, and held at 90% until 2.0 minutes % mobile phase A. UV detection was at 220 nm and MS was measured in positive ion mode.
[0241] LC / MS method B...
Embodiment 2
[0244] Embodiment 2: synthetic method A
[0245]
[0246] Piperazine intermediate 1001 is generally commercially available or can be prepared by various literature methods (i.e., Rong Gao and Daniel J. Canney. A versatile and practical microwave-assisted synthesis of sterically hindered N-arylpiperazines, J. Org. Chem. , 2010, 75(21), 7451-53). For example, aniline or aminoheteroaryl starter 1002 can be reacted with bis(2-chloroethyl)amine and sulfolane at 140°C to give intermediate 1001. (Lokesh Ravilla et al., An efficient scale up process for synthesis of N-arylpiperazines Tetrahefron Letters, 2015, 56(30), 4541-44). Alternatively, protected piperazines can be reacted with bromoaryl or bromoheteroaryl compounds 1003 under Buchwald conditions to afford the desired intermediate 1001.
[0247] Using KF as a basic catalyst and heating in DMSO, 1001 undergoes a nucleophilic substitution reaction with 5-bromopyrimidine-2,4(1H,3H)-dione 1004 to give 5-piperazinylpyrimidine ...
Embodiment 3
[0293] Embodiment 3: synthetic method B
[0294] According to Synthetic Method B, the compounds of the present invention can be prepared by Suzuki or Stille coupling reactions as shown below.
[0295]
[0296] Alternatively, the bromophenyl derivative 1010 can also be converted to the boronic acid ester 1011 shown below, which can then be reacted under Suzuki reaction conditions with a variety of aryl or heteroaryl halides, as shown below with 4 -Chloro-2-methylpyrimidine reaction is exemplified to give the final target as 1012.
[0297]
[0298] Synthesis method B is exemplified by the synthesis of 5-(4-(3-(2-methylpyrimidin-5-yl)phenyl)piperazin-1-yl)pyrimidine-2,4-diamine (compound 69):
[0299]
[0300] 5-(4-(3-bromophenyl)piperazin-1-yl)pyrimidine-2,4-diamine (compound 1010) (1.0g, 2.8mmol, 1.0 equivalent), (2-methylpyrimidine- 5-yl)boronic acid (394.9mg, 2.8mmol, 1.0eq), Cs 2 CO 3 (1.4g, 4.3mmol, 1.5 equivalents), Pd(PPh 3 ) 4 (165.4mg, 143.2umol, 0.05 e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com