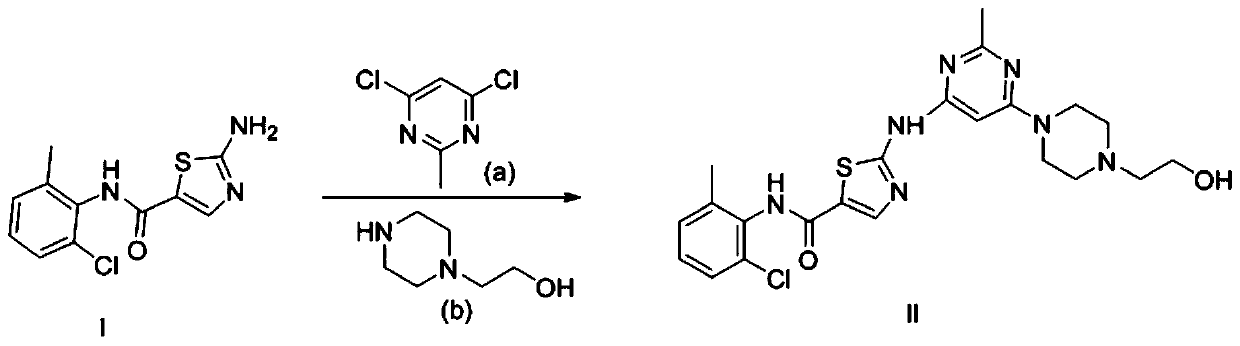
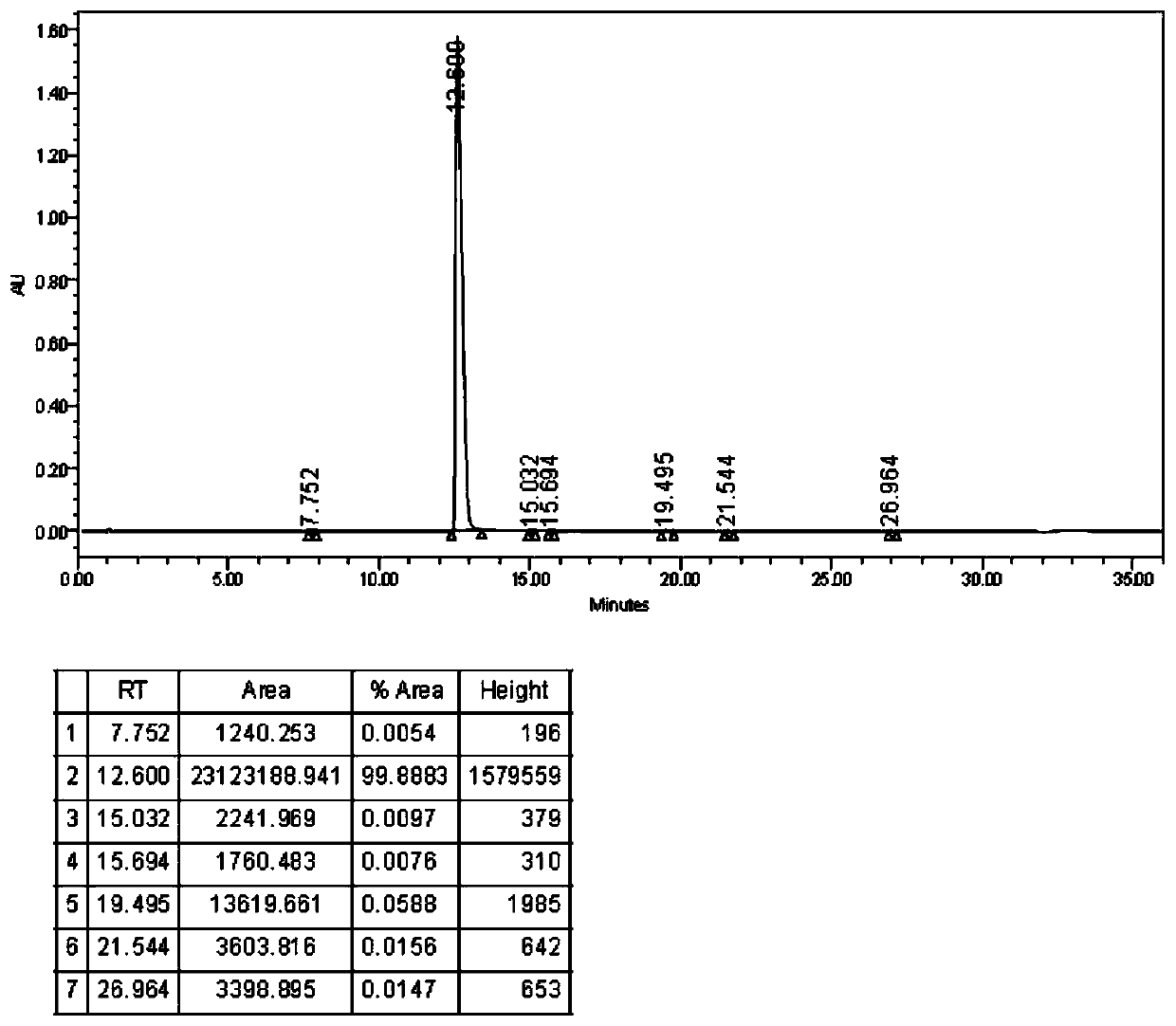
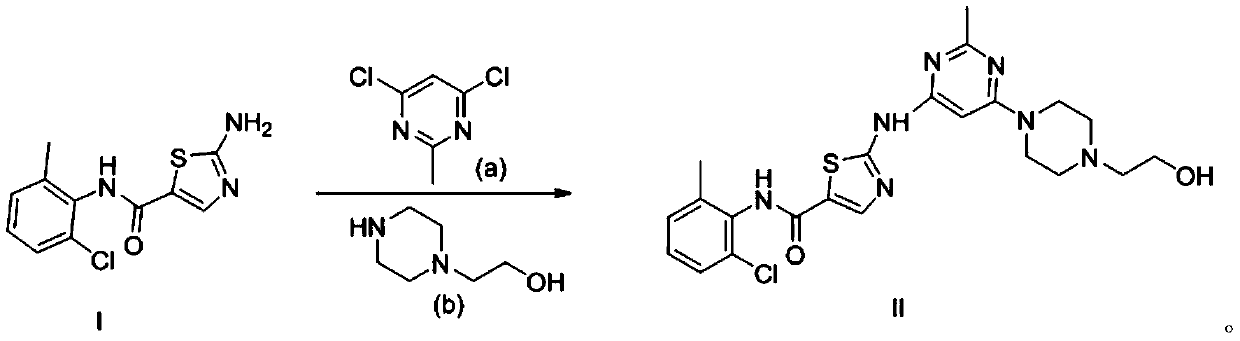
Preparation method of dasatinib hydrate
A technology of dasatinib and hydrate, applied in the field of drug synthesis, can solve the problems of harsh reaction conditions of dasatinib, unsuitable for large-scale production, and difficult to purify, and achieves avoiding separation and purification steps, avoiding waste, and reducing production. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The implementation of the present invention will be illustrated by specific specific examples below, and those skilled in the art can easily understand other advantages and effects of the present invention from the contents disclosed in this specification.
[0030] Before further describing the specific embodiments of the present invention, it should be understood that the protection scope of the present invention is not limited to the following specific specific embodiments; it should also be understood that the terms used in the examples of the present invention are to describe specific specific embodiments, It is not intended to limit the protection scope of the present invention. The test methods for which specific conditions are not indicated in the following examples are usually in accordance with conventional conditions, or in accordance with the conditions suggested by each manufacturer.
[0031] When the examples give numerical ranges, it should be understood t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com