Green synthesis method of sulfadiazine
A technology of sulfadiazine and green synthesis, applied in the direction of organic chemistry, can solve the problems of high cost of environmental protection treatment, high difficulty in the treatment of high-phosphorus organic waste liquid, and secondary environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
preparation example Construction
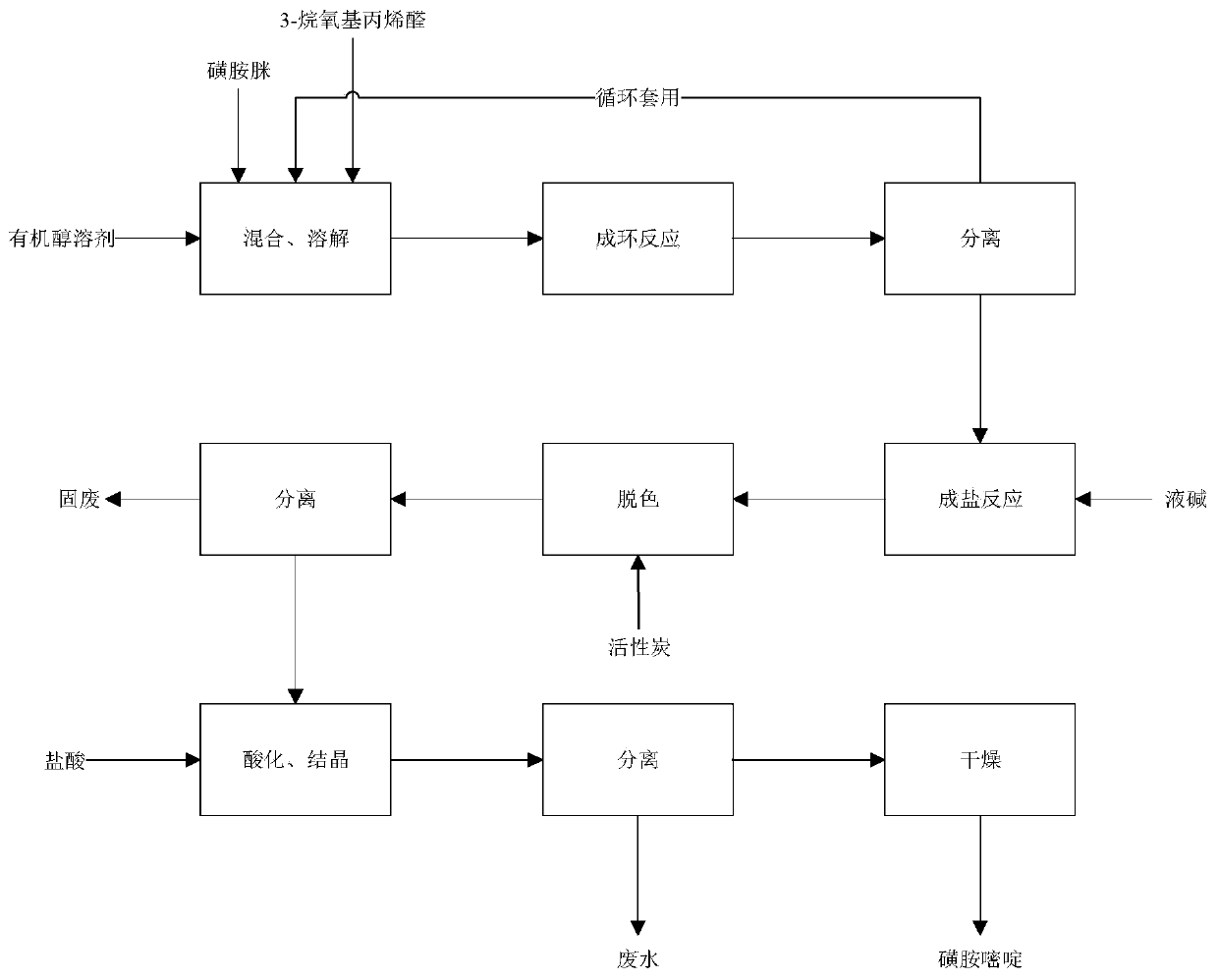
[0019] The green synthetic method of sulfadiazine of the present invention, as figure 1 and figure 2 As shown, it includes the following steps: (a) adding 3-alkoxyacrolein (3-alkoxyacrolein is 3-methoxyacrolein or 3-ethoxypropenal) to the mixture of organic alcohol solvent and sulfamidine Acrolein), after mixing, heat up and reflux to carry out the ring-forming reaction; the chemical structure of the organic alcohol is R—OH, where R is a C1~C4 alkyl group; the temperature of the ring-forming reaction is 50°C~ 140°C; the molar ratio of the sulfamidine to the 3-alkoxyacrolein is 1:1~1.2; (b) after the completion of the cyclization reaction, cool down the mixed material for solid-liquid separation, and obtain The solid is crude sulfadiazine, and the obtained liquid is recycled; (c) adding the crude sulfadiazine to the liquid caustic soda, raising the temperature to carry out a salt-forming reaction, then stirring and decolorizing, and filtering to obtain the filtrate; (d) dripp...
Embodiment 1
[0023] The present embodiment provides a kind of green synthetic method of sulfadiazine, and it comprises the following steps:
[0024] (a) Under normal pressure, add 100g of methanol into the reactor, then add 21.42g of sulfamidine, stir evenly, add 10.33g of 3-methoxyacrolein, stir evenly, raise the temperature to 100°C for reflux and continue to keep warm for 4 hours;
[0025] (b) After the reaction, lower the temperature to 40°C, separate the solid to obtain the crude sulfadiazine, and the liquid is a mixture of solvent and unreacted raw materials (for recycling);
[0026] (c) Add crude sulfadiazine (in step (b)) to 100g of liquid caustic soda, raise the temperature to dissolve the sodium salt of sulfadiazine, and raise the temperature to 70°C to form a salt; add activated carbon, stir for 30 minutes to decolorize, filter to remove the activated carbon to obtain the filtrate ;
[0027] (d) Add 30% hydrochloric acid dropwise to the filtrate until the pH is 5.1~5.4, and car...
Embodiment 2
[0029] This example provides a green synthesis method of sulfadiazine, which is basically the same as that in Example 1, except that: in step (a), 12 g of 3-ethoxyacrolein is added, and the organic alcohol solvent is ethanol; finally The yield of the high-purity sulfadiazine product is 85%, and the purity is 99.2%; after the reaction solution is applied mechanically, the yield is 91%, and the purity is 98.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com