Sodium alginate chewable tablets and preparation method thereof
A technology of sodium alginate and chewable tablets, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, food science, etc., can solve problems such as hidden safety hazards, inability to meet and apply to various groups of people, and avoid excessive aluminum elements. , the preparation method is simple, and the effect is easy to carry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
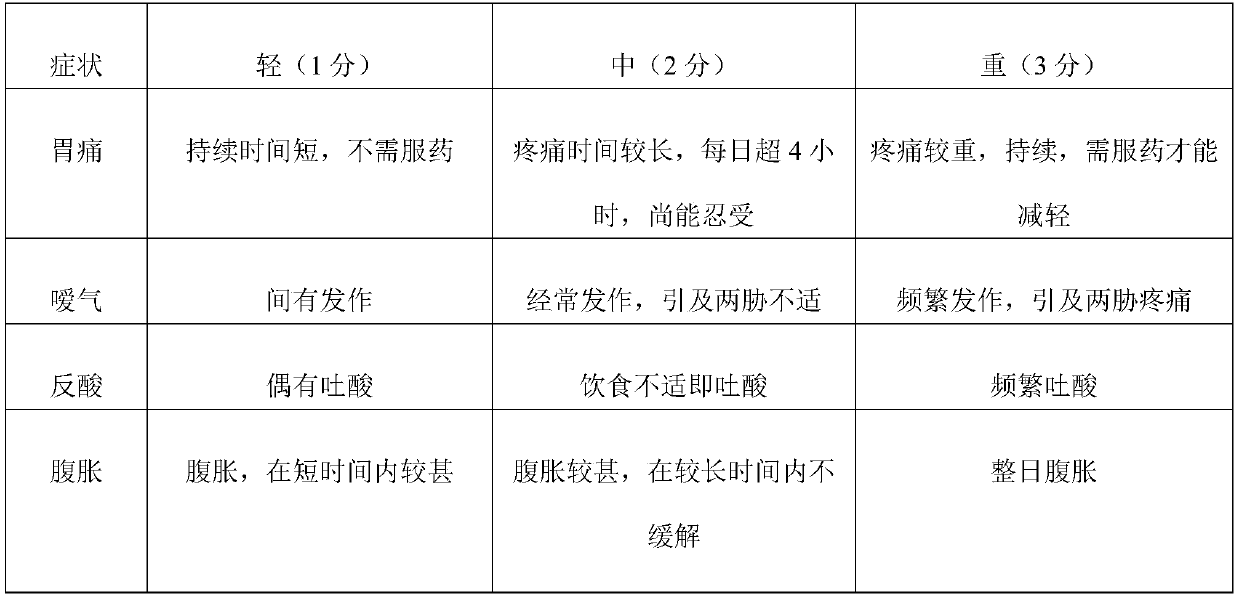
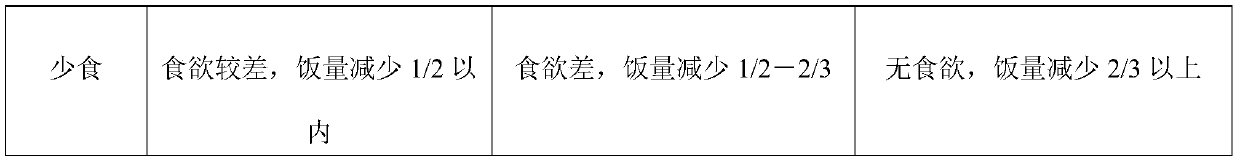
[0036] A sodium alginate chewable tablet, comprising the following components by weight (mg): sodium alginate 200, sodium bicarbonate 300, calcium carbonate 150, magnesium oxide 300, D-mannitol 50, polyethylene glycol 6000 200, Hydroxypropyl Methyl Cellulose E50 5, Mint Flavor 10, Acesulfame K 1, Aspartame 3, Xylitol DC Grade 5, Magnesium Stearate 20.
[0037] A preparation method of sodium alginate chewable tablet, comprising the following steps:
[0038] (1) Weighing of raw materials: weigh each component according to the formula quantity, and set aside;
[0039] (2) Granulation:
[0040] (21) Polyethylene glycol 6000, hydroxypropyl methylcellulose and purified water are mixed into 20% and 5% concentration solutions, and used as adhesive for subsequent use;
[0041] (22) Mix the formula amount of sodium alginate, sodium bicarbonate, calcium carbonate, D-mannitol, acesulfame K, and aspartame evenly, add binder, and granulate with 30 mesh;
[0042] (3) Drying: dry the prepa...
Embodiment 2
[0047] A sodium alginate chewable tablet, comprising the following parts by weight (mg): sodium alginate 300, sodium bicarbonate 50, calcium carbonate 300, magnesium oxide 30, D-mannitol 300, polyethylene glycol 6000 50, Hydroxypropyl Methyl Cellulose E50 20, Mint Flavor 1, Acesulfame K 3, Aspartame 1, Xylitol DC Grade 100, Magnesium Stearate 5.
[0048] Its preparation method is with embodiment 1.
Embodiment 3
[0050] A sodium alginate chewable tablet, comprising the following components by weight (mg): sodium alginate 225, sodium bicarbonate 120, calcium carbonate 250, magnesium oxide 170, D-mannitol 220, polyethylene glycol 6000 100, Hydroxypropyl Methyl Cellulose E50 12, Peppermint Flavor 3, Acesulfame K 3, Aspartame 1, Xylitol DC Grade 50, Magnesium Stearate 10.
[0051] Its preparation method is with embodiment 1.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap