A kind of preparation method of sulfasalazine impurity d
A technology for sulfasalazine and impurities, which is applied in the field of drug impurity synthesis, can solve problems such as easy residues and difficult removal, and achieve the effects of convenient purification, high reaction efficiency, and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
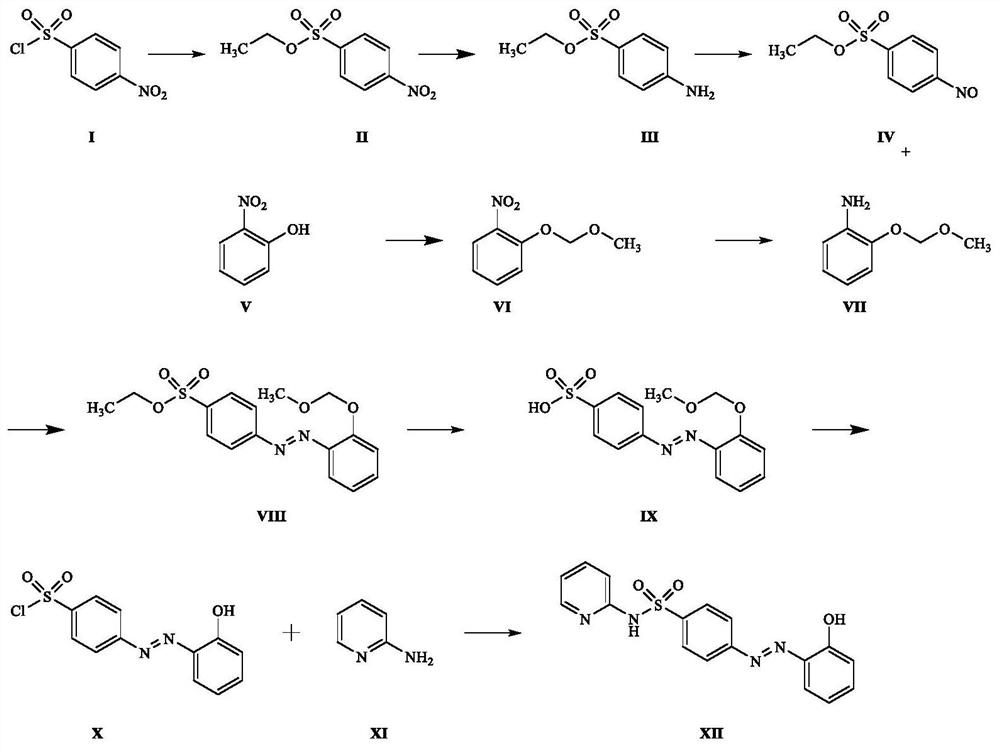
[0041] Such as figure 1 Shown, a kind of preparation method of sulfasalazine impurity D, comprises the following steps:
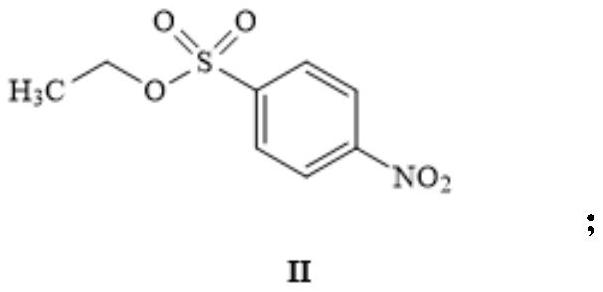
[0042] (1) Dissolve 100.0 g of p-nitrobenzenesulfonyl chloride in 6 L of dioxane, add 110.5 g of sodium ethoxide (prepared from metal sodium and absolute ethanol) at 40° C. and react overnight. TLC spotting shows that the raw materials are completely reacted. Treatment: Add 1L of water and 500mL of ethyl acetate to extract to obtain intermediate II (92.4g, 88%) as a yellow solid;
[0043] (2) Dissolve 50.0g of intermediate II in 500mL of ethanol, add 15.0g of Rey-Ni hydrogenation balloon at room temperature and react overnight, TLC spot plate shows that the reaction of the raw materials is complete, filter the reacted mixture with suction, and spin the filtrate to obtain a yellow solid Intermediate III (42.0 g, 96%);
[0044] (3) Dissolve 20.0g of intermediate III in 500mL of dichloromethane, add 61.1g of mCPBA, and react at 25°C for 2h. TLC spotting shows ...
Embodiment 2
[0052] Such as figure 1 Shown, a kind of preparation method of sulfasalazine impurity D, comprises the following steps:
[0053] (1) Dissolve 100g of p-nitrobenzenesulfonyl chloride in 3L of THF, add 153.5g of sodium ethoxide (prepared from metal sodium and absolute ethanol), and react overnight at 60°C. TLC spotting shows that the raw materials have reacted completely, and add 1L of water , 500mL of ethyl acetate was extracted to obtain 100.8g of yellow solid intermediate II, with a yield of 96%;
[0054] (2) Dissolve 50.0g of intermediate II in 1L of ethanol, add 10.0g of palladium-calcium carbonate, and react overnight with a hydrogenation balloon at room temperature. TLC spotting shows that the reaction of the raw materials is complete. The reaction mixture is suction filtered, and the filtrate is spin-dried to obtain a yellow solid intermediate III (43.1 g, 99%);
[0055] (3) Dissolve 20.0g of intermediate III in 500mL of dichloromethane, add 70.5g of peracetic acid, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com