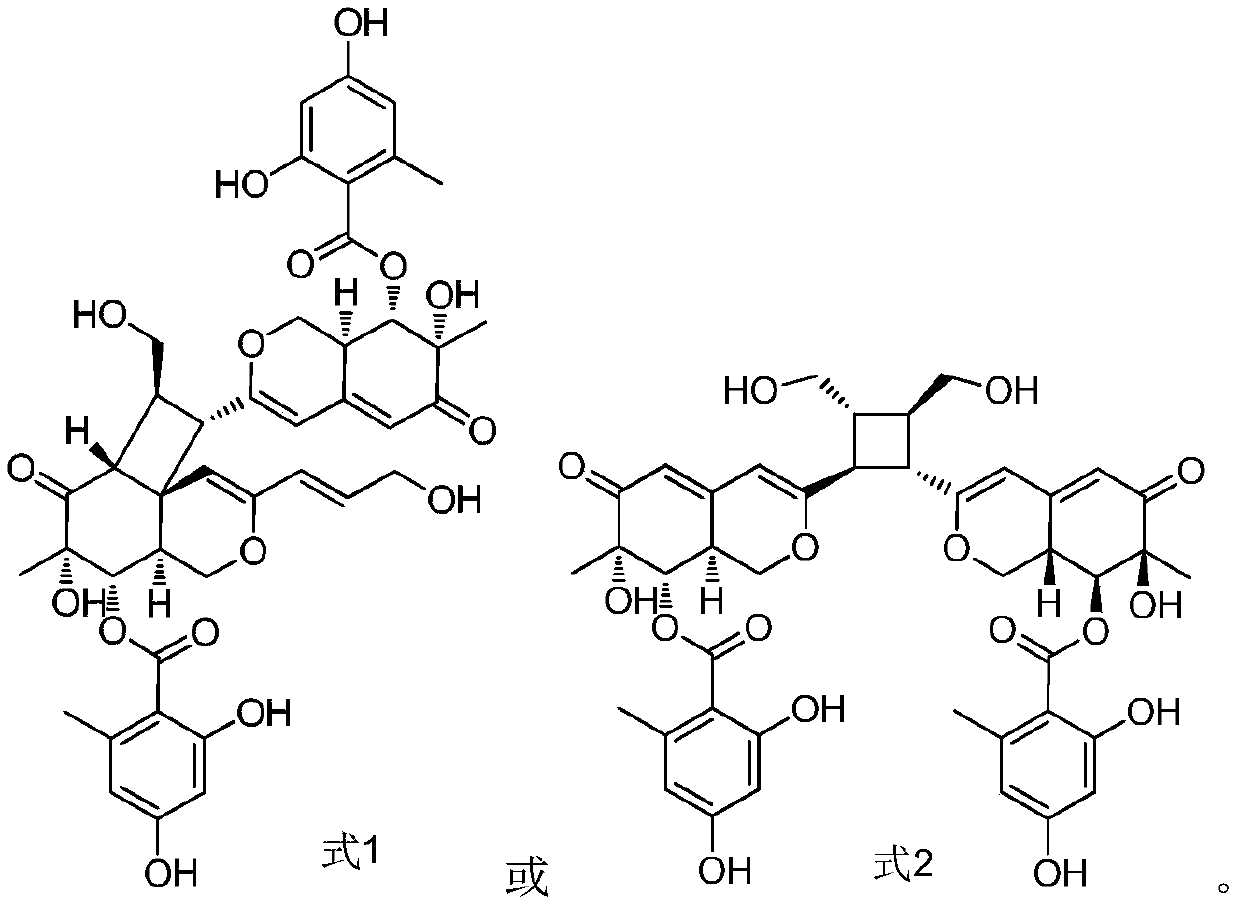
Marine fungus derived azaphilones dimer compound and application thereof in anti-tuberculosis medicaments
A technology of marine fungi and compounds, applied in fungi, antibacterial drugs, microorganism-based methods, etc., can solve the problems of limited species and anti-tuberculosis activity, and achieve the effect of strong inhibitory activity and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
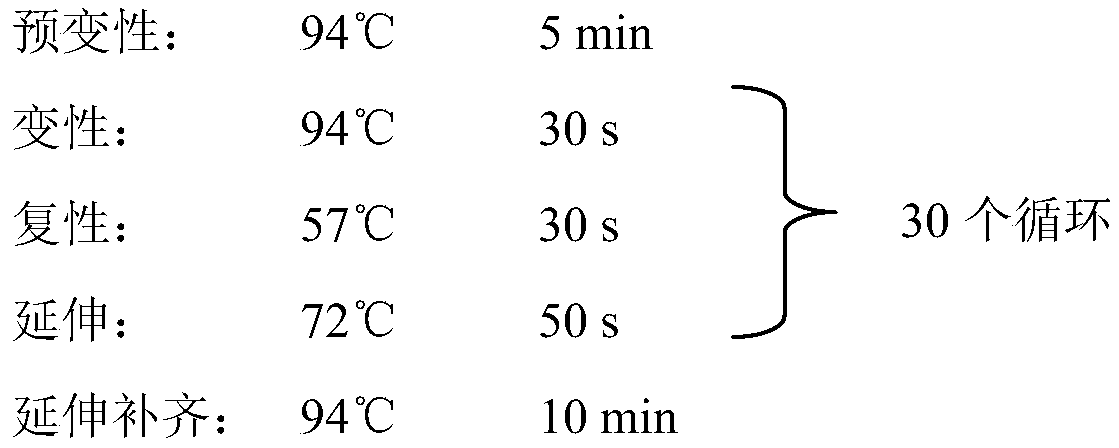
[0049](1) Cultivation of the marine fungus HBU-135 strain
[0050] The medium used for the culture of marine fungus HBU-135 contains 1.0wt% glucose, 0.1wt% yeast extract, 0.2wt% peptone, 1.0wt% agar, 3.0wt% coarse sea salt, and the rest is water. It is made into a test tube when used For slant, the marine fungus strain HBU-135 was grown at 28°C for 3 days.
[0051] (2) Fermentation of marine fungus HBU-135
[0052] The fermentation medium used for the fermentation culture of the marine fungus HBU-135 is that each 1000mL Erlenmeyer flask contains 100g of potatoes (boiled in water for 20 minutes to remove the potato dregs), 20g of glucose, and CaCl 2 33g and 500mL of water, the strain was fermented and cultured on a shaking table at 28°C for 14 days to obtain a fermented product; a total of 20 1000mL Erlenmeyer flasks were used for fermentation.
[0053] (3) Separation and analysis of Azaphilones dimers
[0054] Take the fermented product obtained in step (2), extract 3 time...
Embodiment 2
[0059] (1) Cultivation of the marine fungus HBU-135 strain
[0060] The medium used for the culture of the marine fungus HBU-135 contains 10wt% of glucose, 4.0wt% of yeast extract, 4.0wt% of peptone, 6.0wt% of agar, 10wt% of coarse sea salt, and the rest is water. Fungal strains were grown at 35°C for 5 days.
[0061] (2) Fermentation and cultivation of marine fungus HBU-135
[0062] The fermentation medium used for the fermentation culture of the marine fungus HBU-135 is that each 1000mL Erlenmeyer flask contains 120g of potatoes (boiled in water for 20 minutes to remove the potato dregs), 30g of glucose, and CaCl 2 40g, 600mL of water; the fermentation conditions are 20 days at 35°C on a shaking table to obtain a fermented product.
[0063] (3) Separation and analysis of Azaphilones dimer compounds
[0064]Take the fermented product obtained in step (2), extract 4 times with ethyl acetate, combine the ethyl acetate extracts and concentrate under reduced pressure to obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Minimum inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com