Sodium salt, crystal form and preparation method of pyrrolidine carboxylic acid compound
A compound and sodium salt technology, applied in the field of medicinal chemistry, can solve the problems of easy degradation and discoloration, poor stability, aggravated degradation and racemization, etc., and achieve good curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
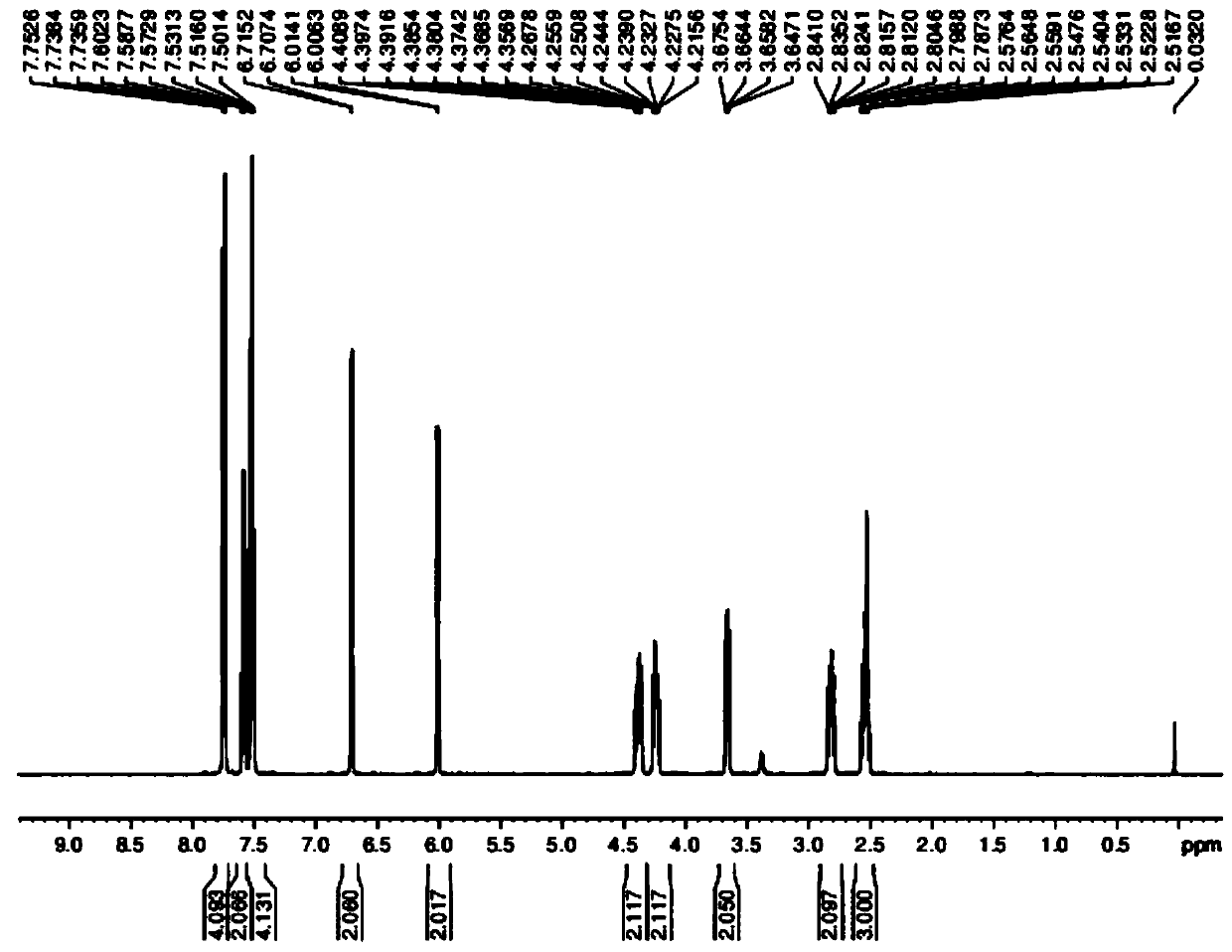
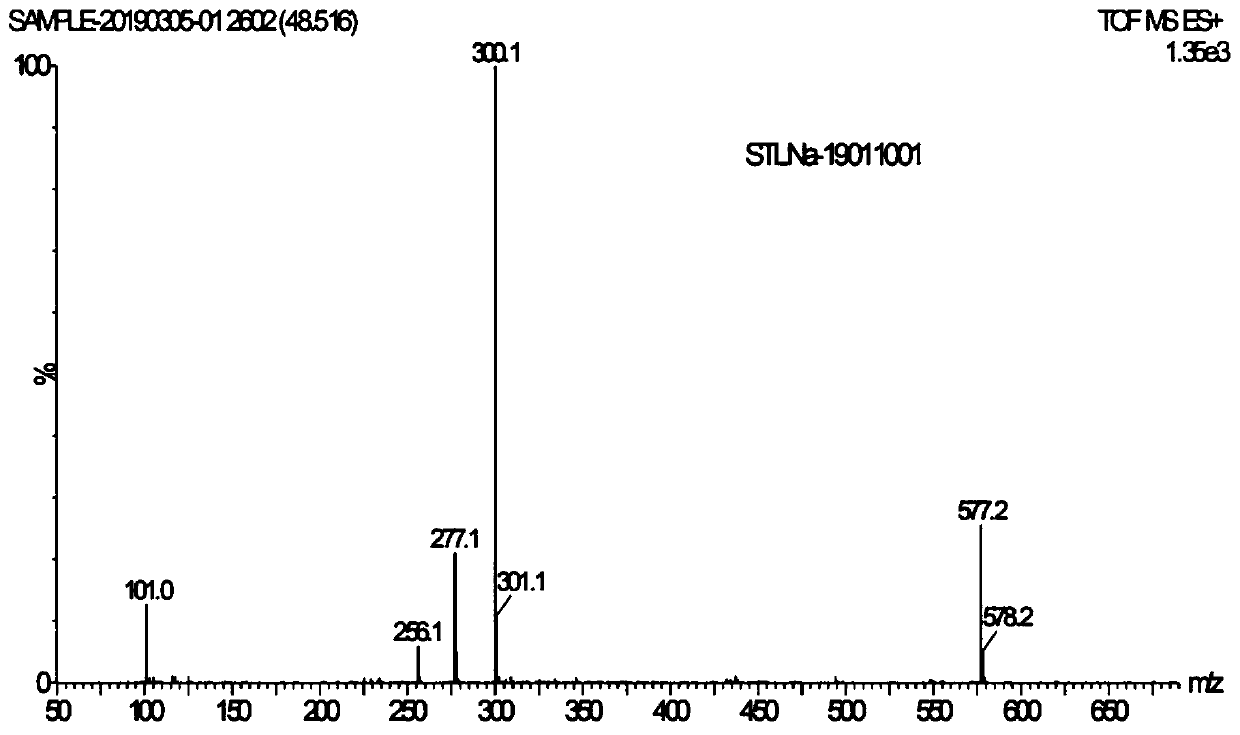
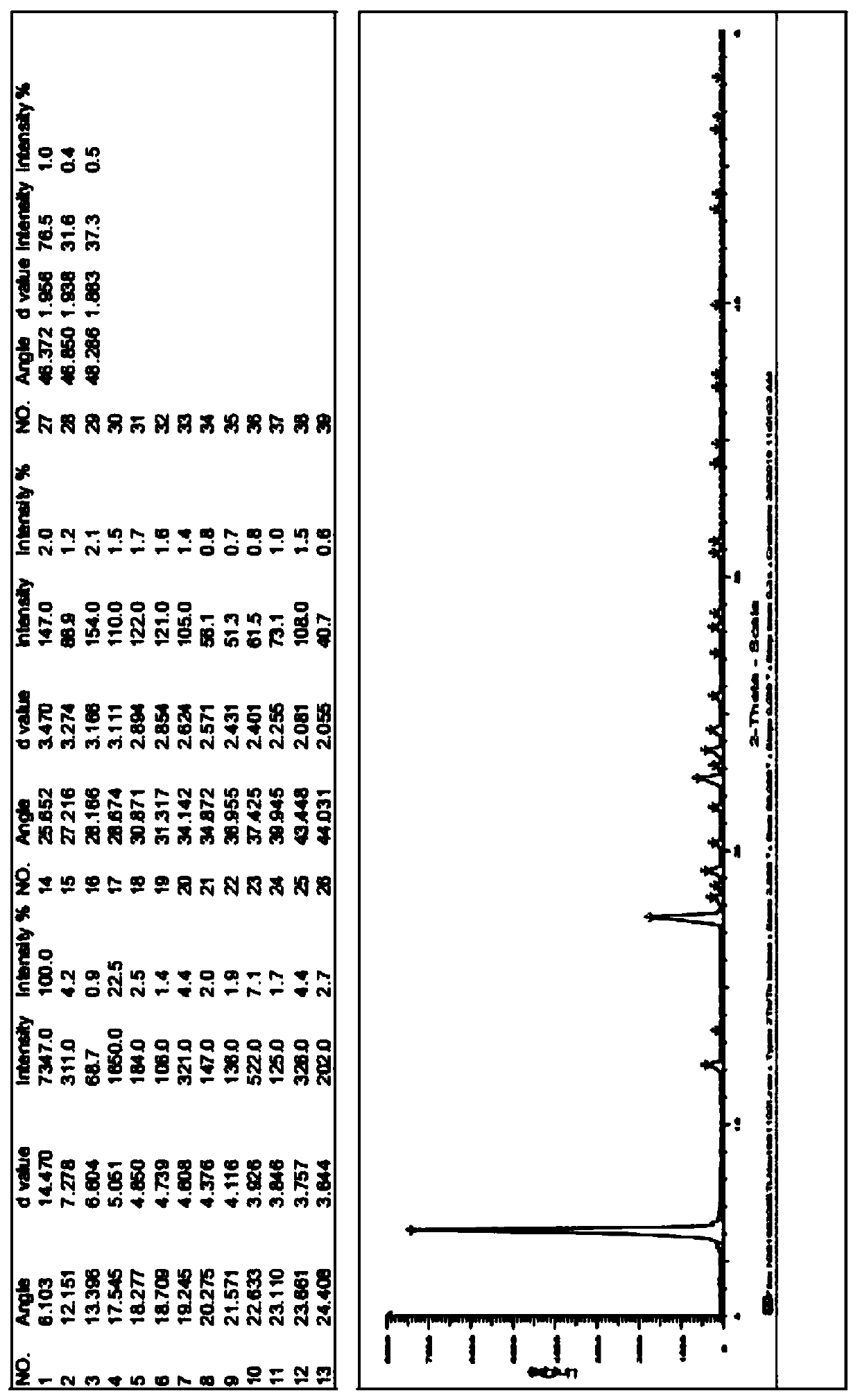
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 formula II compound
[0055] The structure of the compound of formula II is shown below:
[0056]
[0057] Formula II compound is the preparation method of the sodium salt of formula I compound, comprises the following steps:
[0058] Step 1: Add 100 g of ketorolac, 550 mL of 95% ethanol, and 115 g of octylglucamine into the reaction flask, heat up to an internal temperature of 75° C. under stirring, and keep the reaction for 0.5 h. Slowly cool down to 35°C within 2h, keep stirring for 2h, then cool down to -10°C, keep stirring for 12h, filter, wash the filter cake with 95% ethanol at 0°C, and dry it under reduced pressure at 50°C for 7h to obtain off-white 129 g of the solid was compound 3, and the yield was 60%.
[0059] Step 2, add 129g of compound 3 to 1400mL of purified water and 1000mL of ethyl acetate, adjust the pH value of the system to 6-7 with 4N dilute hydrochloric acid, continue stirring at room temperature for 0.5h, let...
Embodiment 2
[0064] The preparation of embodiment 2 formula II compound
[0065] The structure of the compound of formula II is shown below:
[0066]
[0067] Formula II compound is the preparation method of the sodium salt of formula I compound, comprises the following steps:
[0068] Step 1: Add 100 g of ketorolac, 550 mL of 95% ethanol, and 100 g of octylglucamine into the reaction flask, heat up to 70° C. under stirring, and keep the reaction for 0.5 h. Slowly cool down to 30°C within 2h, keep stirring for 1h, then cool down to -5°C, keep stirring for 12h, filter, wash the filter cake with 95% ethanol at 5°C, and dry under reduced pressure at 45°C for 5h to obtain off-white 125 g of the solid was compound 3, and the yield was 56%.
[0069] Step 2, add 125g of compound 3 to 1400mL of purified water and 1000mL of ethyl acetate, adjust the pH value of the system to 6-7 with 4N dilute hydrochloric acid, continue to stir at room temperature for 0.5h, let stand to separate the liquid, c...
Embodiment 3
[0073] The preparation of embodiment 3 formula II compound
[0074] The structure of the compound of formula II is shown below:
[0075]
[0076] Formula II compound is the preparation method of the sodium salt of formula I compound, comprises the following steps:
[0077] Step 1: Add 100 g of ketorolac, 550 mL of 95% ethanol, and 150 g of octylglucamine into the reaction flask, raise the temperature to 70° C. under stirring, and keep the reaction for 0.5 h. Slowly cool down to 30-35°C within 2h, keep stirring for 1-2h, then cool down to -8°C, keep stirring for 12-15h, filter, wash the filter cake with 95% ethanol at 0°C, and depressurize at 45°C After drying for 5-7 hours, 130 g of compound 3 was obtained as an off-white solid with a yield of 60%.
[0078]Step 2, add 130g of compound 3 to 1500mL of purified water and 1000mL of ethyl acetate, adjust the pH value of the system to 6-7 with 4N dilute hydrochloric acid, continue to stir at room temperature for 0.5h, let stand...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com