Stem cells and devices for bone regeneration
A bone regeneration and stem cell technology, applied in the field of scaffolds, bone regeneration, and 3D printing scaffolds, can solve the problem that the processing resolution cannot meet the requirements of micro-scale feature manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0288] Embodiment 1. mouse CNCC and BMMSC culture
[0289] Four-week-old mice were euthanized according to USC IACUC-approved procedures and their mandibles were isolated, minced and digested with 2 mg / ml type I collagenase and 4 mg / ml dispase II in PBS for 1 hour at 37°C. The long bone marrow was flushed from the femur and tibia, centrifuged quickly and resuspended. Single-cell suspensions were obtained by passing cells through a 70 μm filter and inoculated in a medium supplemented with 20% FBS, 2 mM L-glutamine, 55 μM 2-mercaptoethanol, 100 U / ml penicillin, and 100 μg / ml streptavidin. Prime α-MEM in a 10 cm plate dish. The medium was changed after the initial incubation for 48 hours.
Embodiment 2
[0290] Example 2. Subcutaneous cell implantation
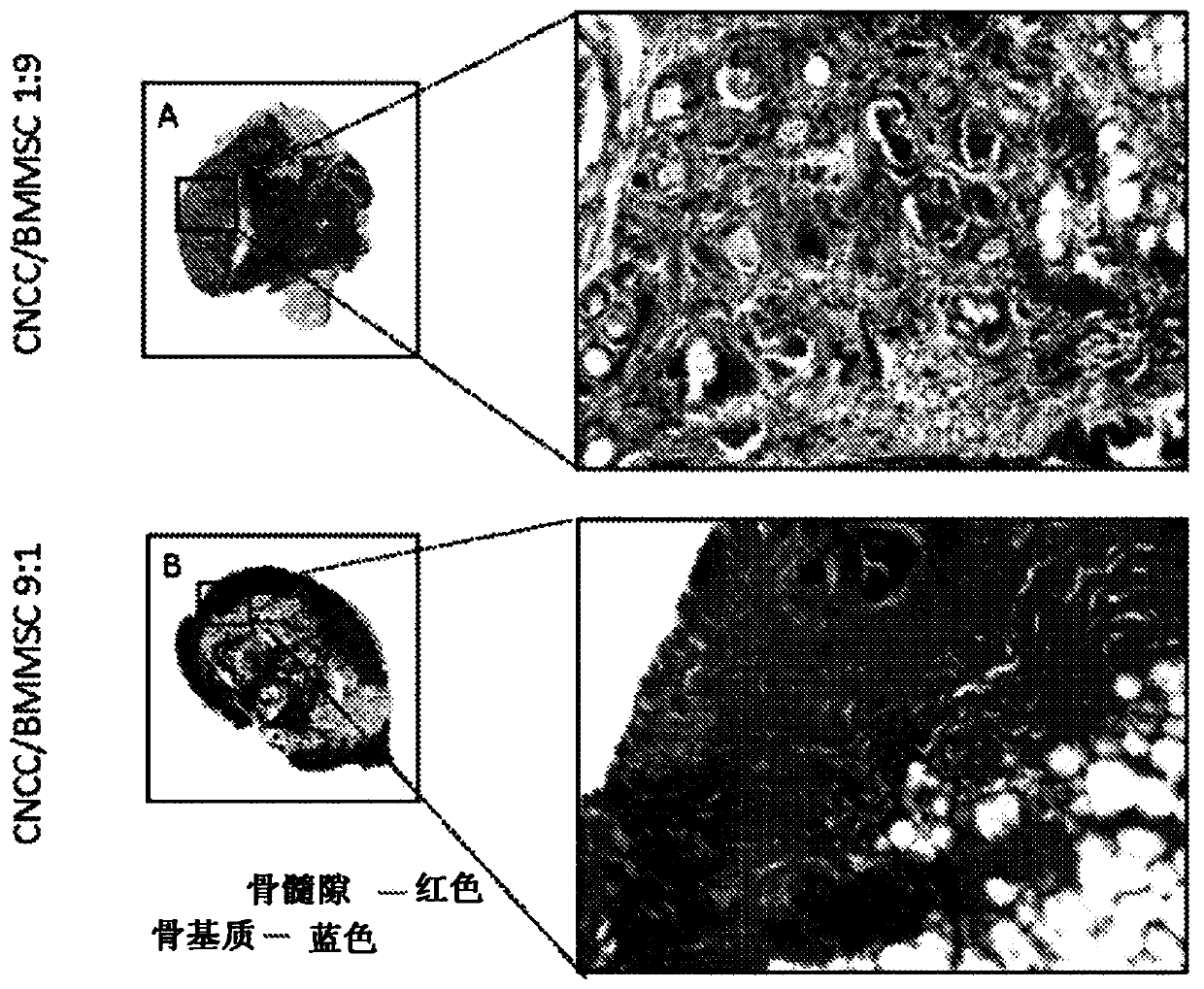
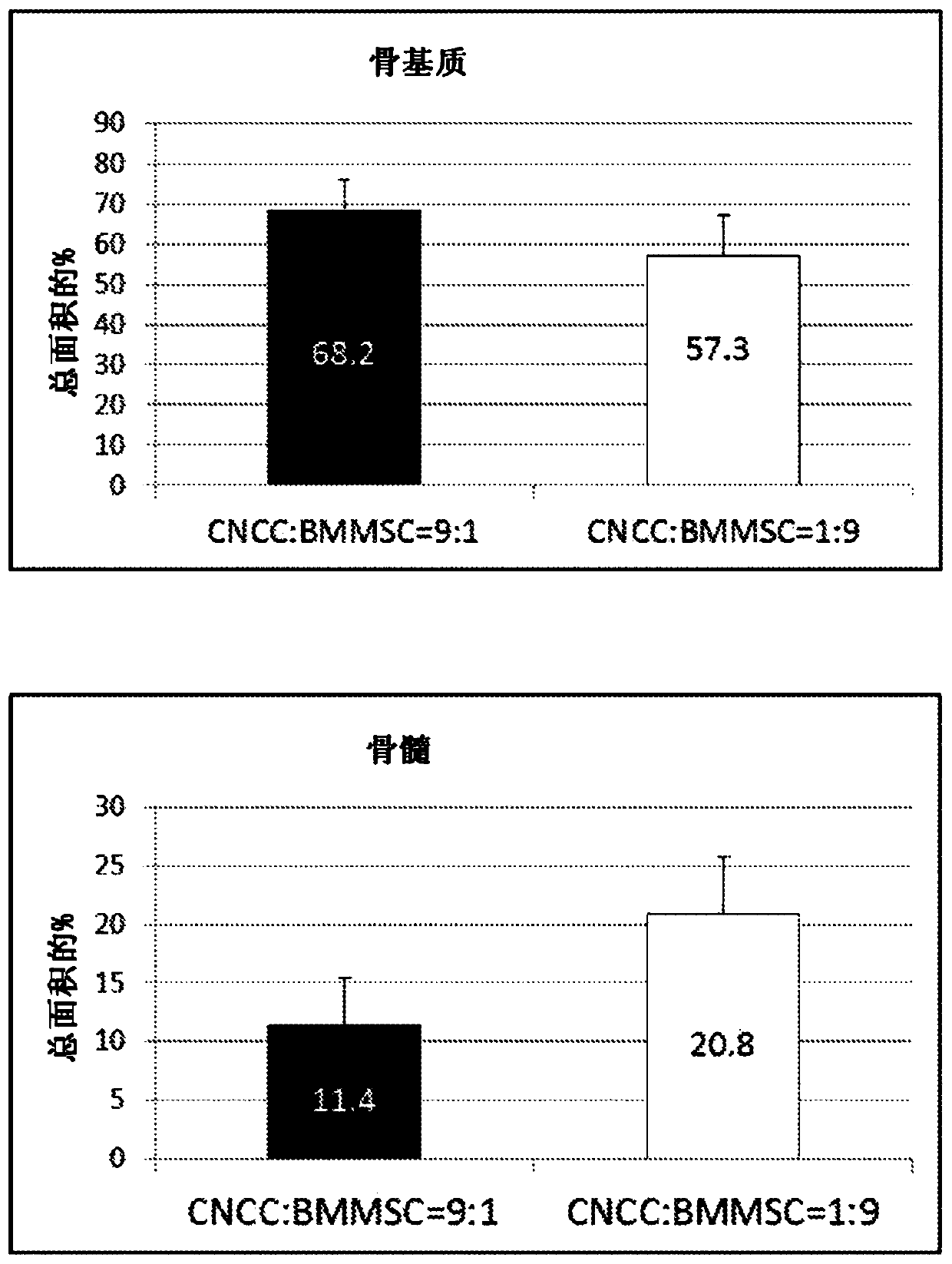
[0291] CNCC and BMMSC were mixed in ratios of 9:1, 5:5 and 1:9. Then add a total of 2×10 6 cells implanted under the skin of immunocompromised mice. Mice were euthanized and cells were harvested three months after implantation, fixed in 4% PFA, decalcified and sectioned for histological examination.
Embodiment 3
[0292] Example 3. Scaffold degradation and cytotoxicity
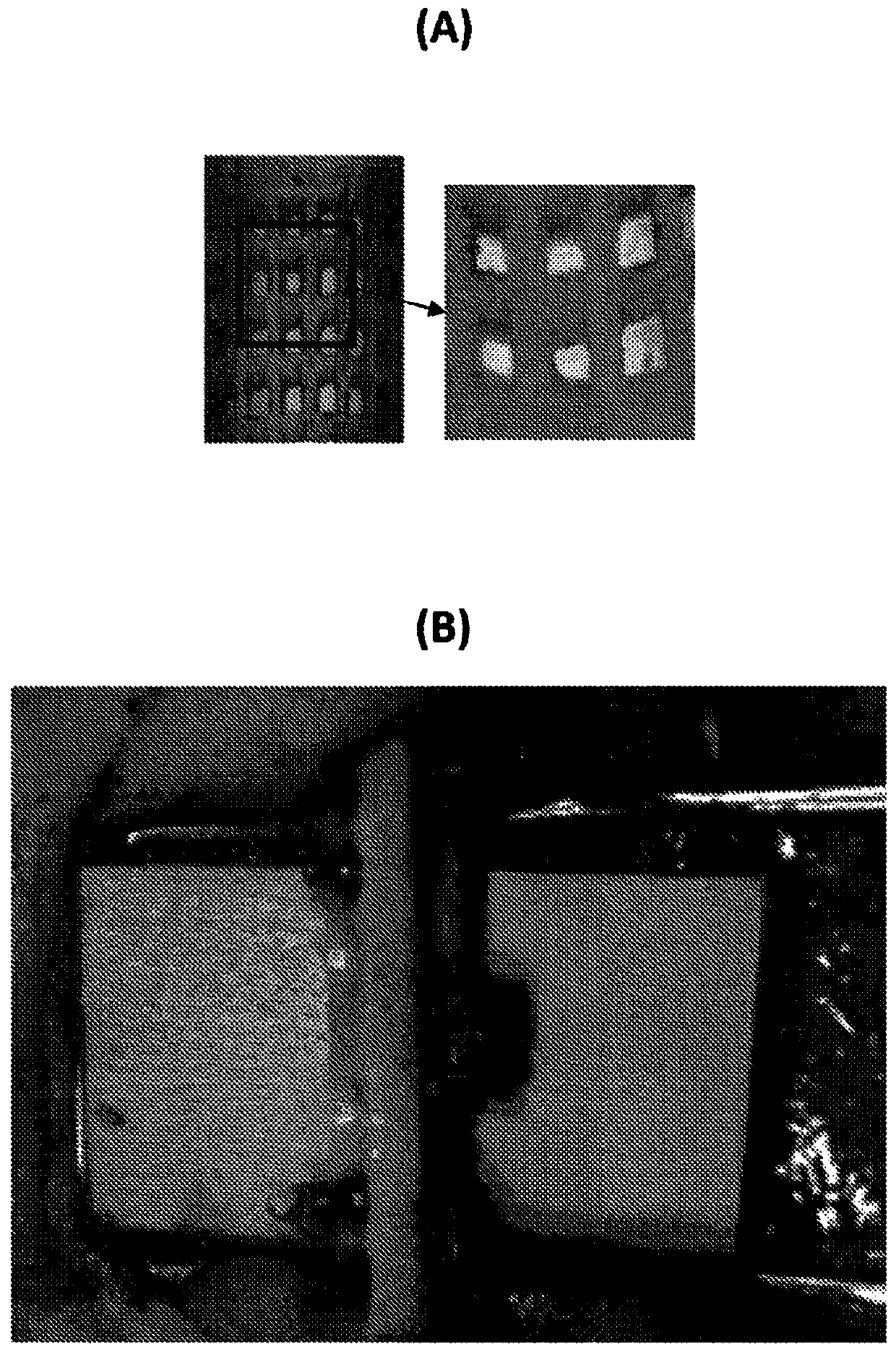
[0293] Polycaprolactone (PCL)-based 3D-printed scaffolds were implanted subcutaneously in immunocompromised mice and collected at various time points to test their degradation profile by measuring their dry weight.
[0294] To test the potential cytotoxicity of PCL scaffolds, NIH3T3 cells were incubated at 1 × 10 4 Cells / well were seeded in 96-well plates. After 24 hours of incubation to allow cell attachment, the PCL scaffolds were co-cultured in DMEM medium with 10% FBS for 72 hours. MTT cell assay to test cell viability was performed following the manufacturer's instructions (Abeam).
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com