Benzocoumarin chalcone neuraminidase inhibitor and preparation method and application thereof
A technology of neuraminidase and benzocoumarin, applied in antiviral agents, organic chemistry, etc., can solve the problems of complex synthesis process and expensive raw materials for Tamiflu production, and achieve simple synthesis method and neuraminidase inhibition active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
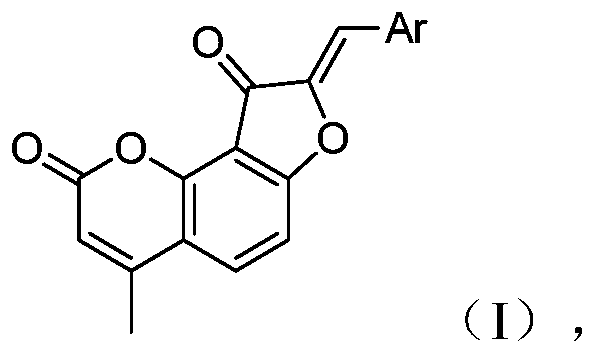
[0029] A preparation method of benzocoumarin chalcone neuraminidase inhibitor, its structural formula is as shown in formula 1:
[0030]
[0031] Concrete synthetic steps are as follows:
[0032] (1) Accurately weigh 5.5g (50mmol) of resorcinol in a 50ml round bottom flask, add 15ml of 2,4-dioxane, stir and dissolve in an ice bath, then slowly add 2ml of concentrated sulfuric acid dropwise, and then use Add 7ml (55mmol) ethyl acetoacetate dropwise to the constant pressure dropping funnel. After the dropwise addition, take out the round-bottomed flask, place it in a constant temperature oil bath at 60°C, heat and stir for 4 hours, take it out, pour the reaction solution into 100ml of ice water and stir for 30 minutes, and let stand to precipitate a large amount of precipitate. After filtering, the filter cake was washed several times with a large amount of ice water, dried in a vacuum oven, and recrystallized from methanol to obtain the intermediate formula II.
[0033] (2...
Embodiment 2
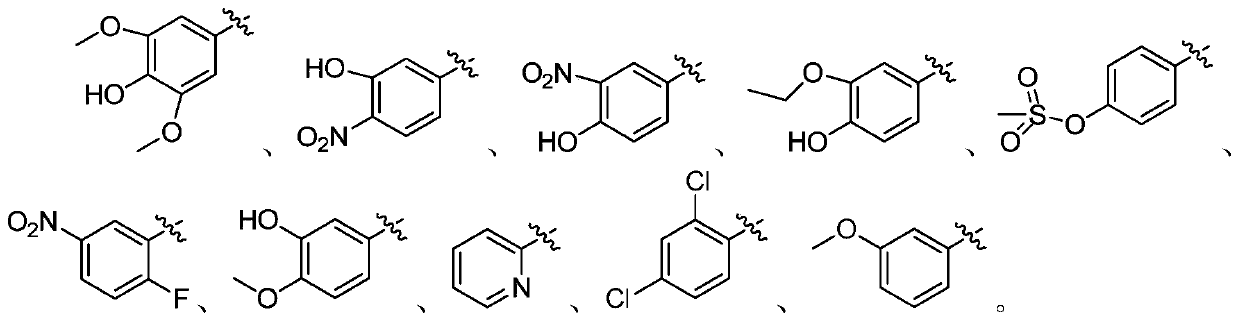
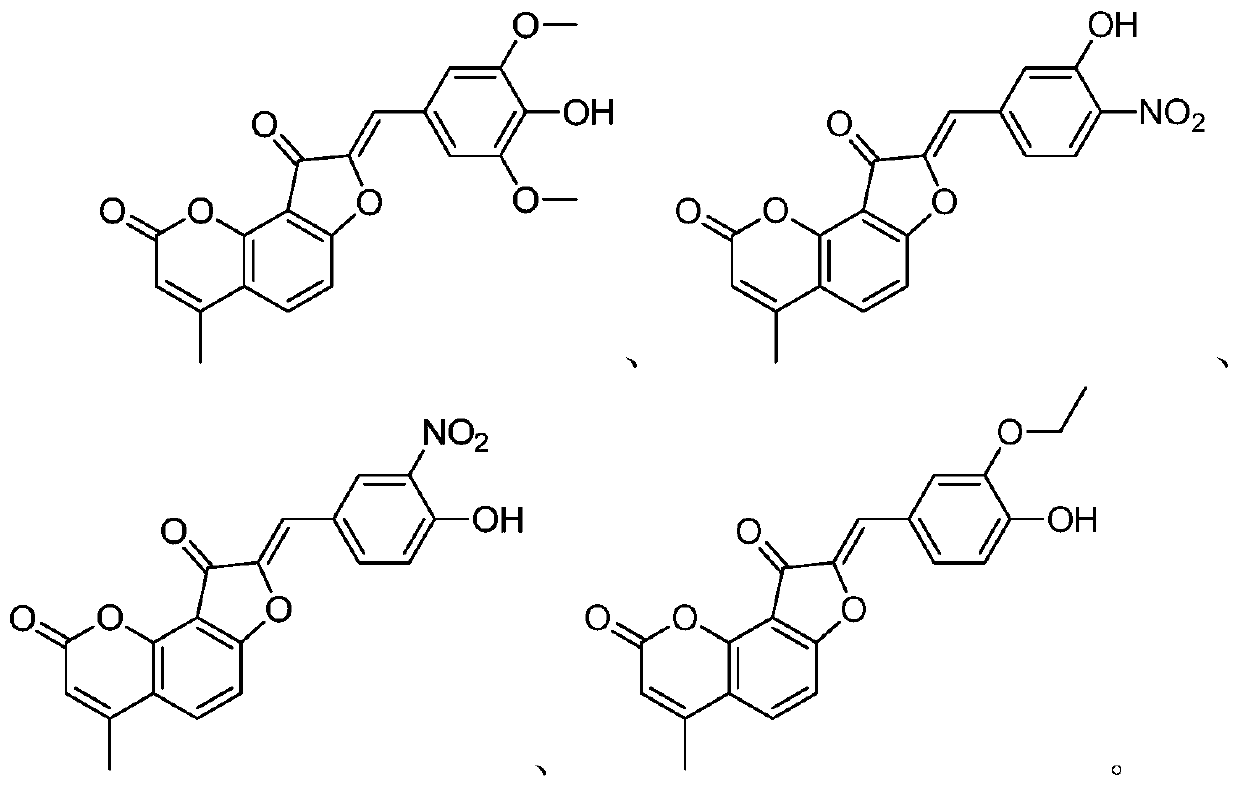
[0041] A preparation method of a benzocoumarin-chalcone neuraminidase inhibitor, whose structural formula is as follows, prepared by a method similar to Example 1.
[0042] (Z)-8-(3-Hydroxy-4-nitrobenzylidene)-4-methyl-2H-furo[2,3-h]methylene-2,9(8H)-dione
[0043]
[0044]Yellow solid, 58% yield, IC 50 The value is 0.045 μM.
[0045] 1 H NMR (500MHz, CDCl 3 :F 3 CCOOD=3:2)δ8.23(ddd,J=22.5,8.6,2.0Hz,2H),7.88(d,J=1.9Hz,1H),7.56(td,J=6.0,2.9Hz,2H), 7.13(s,1H),6.57(s,1H),2.63(s,3H).
[0046] 13 C NMR (125MHz, CDCl 3 :F 3 CCOOD=3:2)δ182.59,168.68,163.24,156.25,154.47,150.10,148.45,139.96,135.83,133.82,125.65,123.66,122.53,117.52,115.27,113.01,1110.86
Embodiment 3
[0048] A preparation method of a benzocoumarin-chalcone neuraminidase inhibitor, whose structural formula is as follows, prepared by a method similar to Example 1.
[0049] (Z)-8-(4-Hydroxy-3-nitrobenzylidene)-4-methyl-2H-furo[2,3-h]methylene-2,9(8H)-dione
[0050]
[0051] Yellow solid, 55% yield, IC 50 The value is 0.85 μM.
[0052] 1 H NMR (501MHz, DMSO-d 6 )δ8.53(s,1H),8.17(d,J=7.9Hz,2H),7.53(s,1H),7.26(d,J=8.6Hz,2H),7.07(s,1H),6.42( s,1H),2.48(s,3H). 13 C NMR (126MHz, CDCl 3 :F 3 CCOOD=3:2) δ182.47, 168.29, 163.47, 156.66, 156.38, 150.11, 146.73, 140.65, 135.22, 133.68, 129.00, 124.00, 121.03, 117.49, 115.24, 112.96, 1110.76
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com