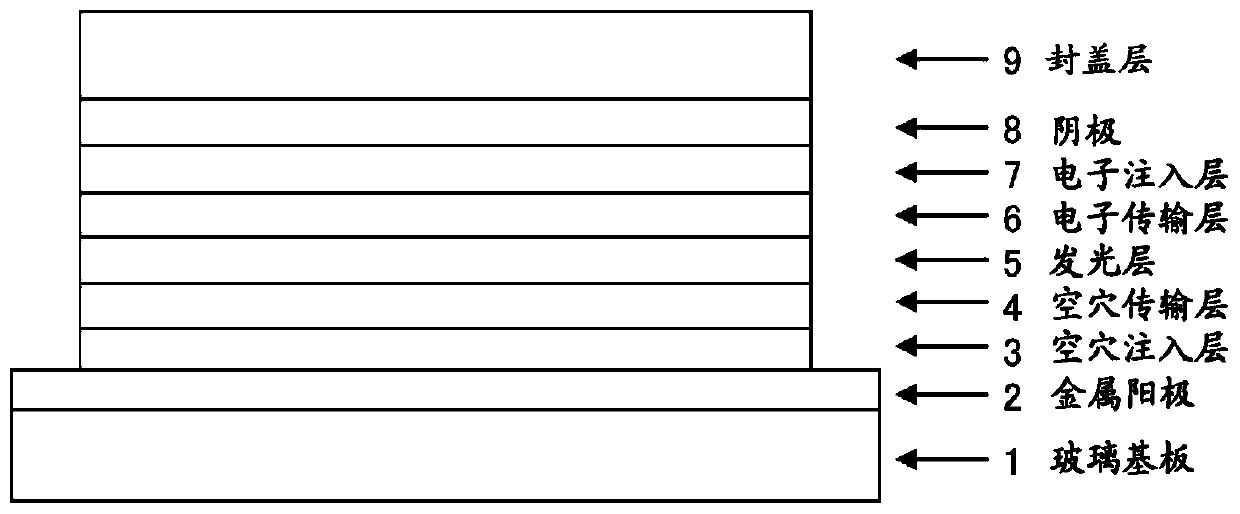
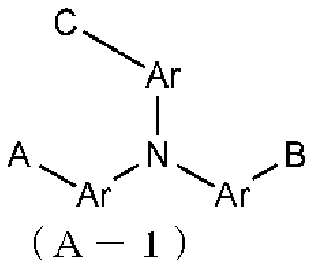
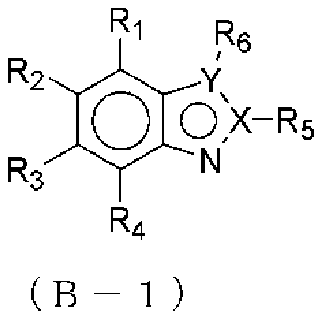
Organic el element, amine compound having benzoazole ring structure, and method in which said amine compound is used in capping layer of organic el element
A technology of benzoxazole ring and capping layer, applied in organic chemistry, organic semiconductor devices, electrical components, etc., can solve the problems of reduced light extraction efficiency, reduced alignment accuracy, deformation of metal masks, etc., and achieves the highest extraction efficiency. The effect of optimization, improved extraction efficiency, and excellent light resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0136]
[0137] Add 2-(4-bromophenyl)-2H-benzotriazole: 3.7g, bis-(biphenyl-4-yl)-{4-(4,4,5, 5-Tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl}-amine: 9.4g, toluene: 80mL, ethanol: 20mL, then, add pre-made potassium carbonate: 5.6 g dissolved in H 2 O: an aqueous solution in 20 mL, nitrogen gas was passed through while irradiating ultrasonic waves for 30 minutes. Tetrakis(triphenylphosphine)palladium: 0.8 g was added, and the mixture was stirred under heating and reflux for 4 hours. After natural cooling, add H 2 O, the organic layer was extracted by liquid separation, and concentrated under reduced pressure to obtain a crude product. Purify the obtained crude product by column chromatography (carrier: silica gel, eluent: toluene / n-hexane) to obtain {4'-(benzotriazol-2-yl)biphenyl-4-yl}-bis- Yellow powder of (biphenyl-4-yl)-amine (compound 17): 6.4 g (85% yield).
[0138] [chemical 20]
[0139]
[0140] The structure of the obtained yellow powder was identified using N...
Embodiment 2
[0144]
[0145] Add 2-(4-bromophenyl)-2H-benzotriazole: 9.0 g, bis-(biphenyl-4-yl)-amine: 9.6 g, sodium tert-butoxide: 4.3 g, Toluene: 150 ml, while irradiating ultrasonic waves for 30 minutes, blow nitrogen gas. Palladium(II) acetate: 0.1 g and a 50% (w / v) toluene solution of tri-(tert-butyl)phosphine: 0.24 ml were added, and the mixture was stirred under reflux for 2 hours. After natural cooling, add toluene, carry out dispersion washing, filter the insoluble matter, add silica gel and activated clay to the filtrate, and carry out adsorption and purification. The adsorbent was removed by filtration and the filtrate was concentrated to give crude product. The crude product is purified by crystallization in a mixed solvent of toluene / acetone, and the precipitated solid is obtained to obtain {4-(benzotriazol-2-yl)phenyl}-bis(biphenyl-4-yl)-amine (compound 6) Yellow powder: 11.6 g (75.3% yield).
[0146] [chem 21]
[0147]
[0148] The structure of the obtained yellow p...
Embodiment 3
[0152]
[0153] Add 2-(4-bromophenyl)-2H-benzotriazole: 8.3g, (biphenyl-4-yl)-(9,9-dimethyl-9H-fluoren-2-yl) into the reaction vessel )-amine: 10.0 g, sodium tert-butoxide: 4.0 g, toluene: 150 ml, nitrogen gas was blown while irradiating ultrasonic waves for 30 minutes. Palladium(II) acetate: 0.1 g and a 50% (w / v) toluene solution of tri-(tert-butyl)phosphine: 0.22 ml were added, and the mixture was stirred under reflux for 2 hours. After natural cooling, add toluene, carry out dispersion washing, filter the insoluble matter, add silica gel and activated clay to the filtrate, and carry out adsorption and purification. The adsorbent was removed by filtration and the filtrate was concentrated to give crude product. The crude product was purified by crystallization with acetone, and the precipitated solid was obtained to obtain {4-(benzotriazol-2-yl)phenyl}-(biphenyl-4-yl)-9,9-dimethyl- Yellow powder of 9H-fluoren-2-yl)-amine (compound 7): 12.3 g (80.4% yield).
[0154] [che...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com