A kind of synthetic method of racemic clopidogrel
A technology of racemic clopidogrel and synthesis method, which is applied in the field of drug synthesis, can solve the problems of rising overall cost and high market price, and achieve the effects of stable properties, mild and easy-to-control reaction conditions, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of synthetic method of racemic clopidogrel, concrete steps are as follows:
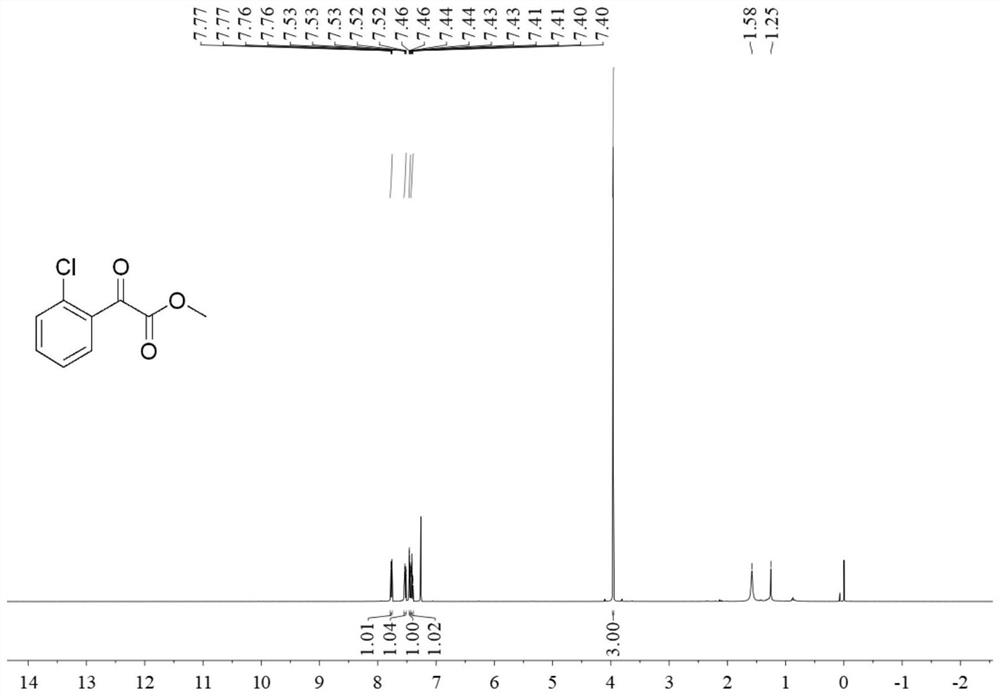
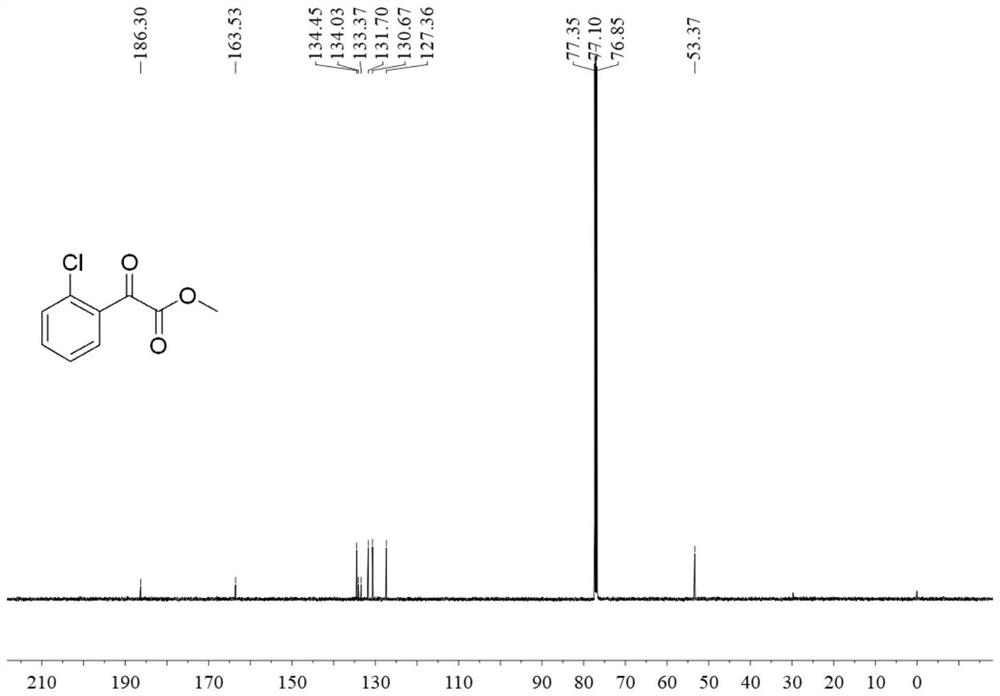
[0033] Step 1: Add 1.5mmol N-chlorosuccinimide, 0.1mmol palladium acetate and 2mL 1,2-dichloroethane to a pressure-resistant reaction tube and mix to obtain a solution, add 1mmol benzoylformate to the solution ester, add 0.2mmol ligand 3,5-bis(trifluoromethyl)aniline, add 10mmol trifluoroacetic acid, react at 80°C for 24 hours, dilute the resulting reaction solution with dichloromethane to 50mL, and then add 50mL saturated bicarbonate Quenched by sodium solution, then extracted three times with dichloromethane, combined the organic phases, added anhydrous sodium sulfate to dry, and removed the solvent by rotary evaporation under reduced pressure at 40 ° C, and carried out column chromatography separation in 200-300 mesh silica gel (eluent Petroleum ether: ethyl acetate = 40:1, v / v) to obtain methyl o-chlorobenzoylformate. The yield of this step is 65%.
[0034] The second step: Add 1.1...
Embodiment 2
[0043] A kind of synthetic method of racemic clopidogrel, concrete steps are as follows:
[0044] The first step: add 1.5mmol N-chlorosuccinimide to the pressure-resistant reaction tube, add 0.1mmol palladium acetate, add 2mL solvent 1,2-dichloroethane, add 1mmol 3-methyl Methyl benzoylformate, add 0.3mmol ligand 3,5-bis(trifluoromethyl)aniline, add 10mmol trifluoroacetic acid, react at 80°C for 24 hours, dilute the obtained reaction solution to 50mL with dichloromethane, Quench with 50 mL of saturated sodium bicarbonate solution, then extract three times with dichloromethane, combine the organic phases, add anhydrous sodium sulfate to dry, remove the solvent by rotary evaporation under reduced pressure at 40°C, and conduct column layer in 200-300 mesh silica gel Analysis and separation (eluent: petroleum ether: ethyl acetate = 40:1, v / v) gave methyl o-chlorobenzoylformate. The yield of this step is 71%.
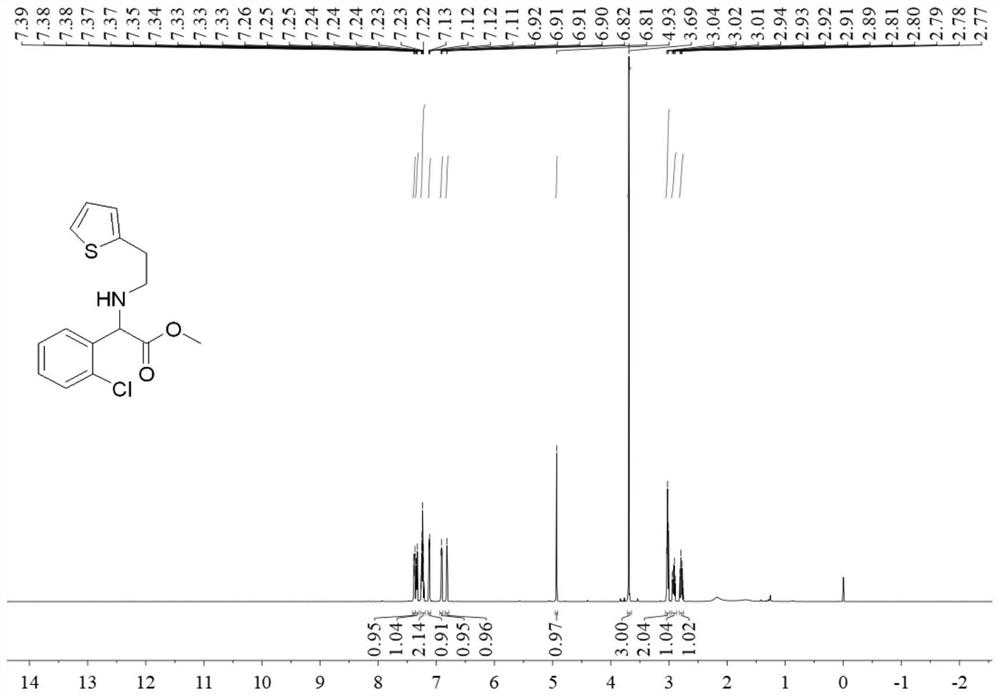
[0045] The second step: Add 1.1 mmol 2-thienylethylamine to 2 mL of d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com