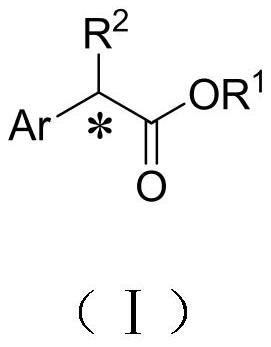
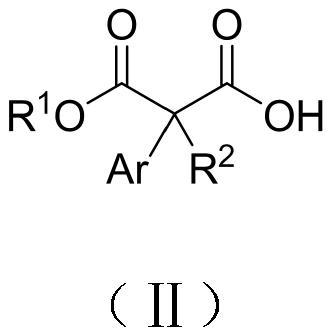
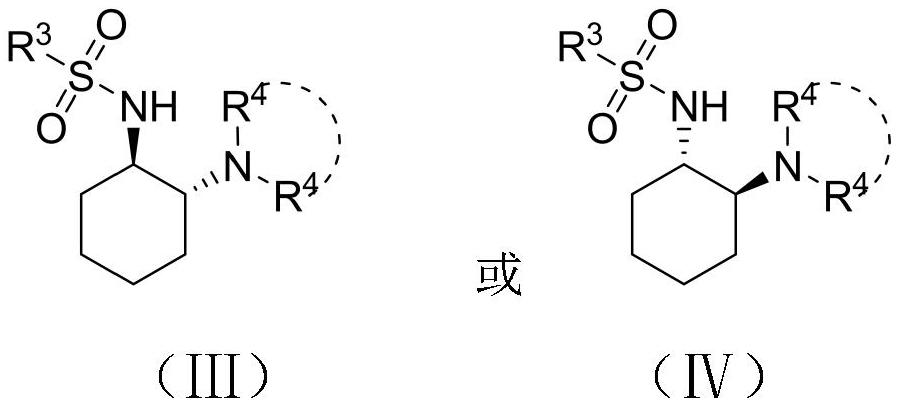
Alpha-alkylphenylacetic acid compound with high optical activity as well as preparation method and application thereof
An optically active, alkylbenzene technology, which is applied in the field of high optically active α-alkylphenylacetic acid compounds and their preparation, can solve the problem that the control of the enantioselectivity of the reaction activity of the catalyst is not very ideal, and achieves that the catalyst is easy to obtain. , the reaction operation is simple, the effect of high enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] The preparation method of highly optically active α-alkylphenylacetic acid compound is as follows:
[0032]
Embodiment 1
[0034] The synthesis of embodiment 1 (R)-2-phenylbutyric acid phenyl ester (2a)
[0035] 2-(Phenoxycarbonyl)-2-phenylbutanoic acid (1a, 28.4mg, 0.1mmol, 1equiv.), N-((1R,2R)-2-(piperidin-1-yl)cyclohexyl) -3,5-Bis(trifluoromethyl)benzenesulfonamide (4.6mg, 0.01mmol, 0.1equiv) was added to a 4mL reaction flask, methyl tert-butyl ether (1.0mL) was added, and the reaction was mixed at 22°C Stir until the reaction of the raw materials is complete; the organic phase is spin-dried, and the remaining residue is purified by silica gel chromatography (petroleum ether / ethyl acetate=20 / 1) to obtain the corresponding product 2a, yield: 98%, ee: 91%. [α] D 20 =-65(c0.23, CHCl 3 ); IR (film) 3414, 2932, 1753, 1610, 1592, 1511, 1252, 816, 750cm -1 ; 1 HNMR (400MHz, CDCl 3 ) , δ7.36–7.29(m,4H),7.22–7.16(m,1H),7.00–6.96(m,2H),6.93–6.87(m,2H),3.81(s,3H),3.64(t,J =7.7Hz,1H),2.26–2.13(m,1H),2.07-1.78(m,1H),0.99(t,J=7.4Hz,3H); 13 CNMR (100MHz, CDCl 3 )δ172.9, 159.0, 150.9, 130.8, 129.5, 1...
Embodiment 2
[0036] The synthesis of embodiment 2 (R)-2-(4-methoxyphenyl) phenyl butyrate (2b)
[0037] Starting from the raw material 1b, the experimental reaction steps were the same as the synthetic method of 2a in Example 1 to obtain the product 2b, the yield: 96%, ee: 90%. [α] D 20 =-65(c0.23, CHCl 3); IR (film) 3414, 2932, 1753, 1610, 1592, 1511, 1252, 816, 750cm -1 ; 1 HNMR (400MHz, CDCl 3 ) , δ7.36–7.29(m,4H),7.22–7.16(m,1H),7.00–6.96(m,2H),6.93–6.87(m,2H),3.81(s,3H),3.64(t,J =7.7Hz,1H),2.26–2.13(m,1H),2.07-1.78(m,1H),0.99(t,J=7.4Hz,3H); 13 CNMR (100MHz, CDCl 3 )δ172.9, 159.0, 150.9, 130.8, 129.5, 129.2, 125.9, 121.6, 114.2, 55.4, 52.8, 26.9, 12.3; HRMS (ESI) CalcdforC 17 h 19 o 3 (M+H): 271.1334.Found: 271.1338.HPLC: ChiralcelOJ-H (25cm×0.46cm), hexane / 2-propanol=90 / 10, 1.0mL / min, 204nm, 25℃, t R =27.54min(minor), 38.92min(major).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap