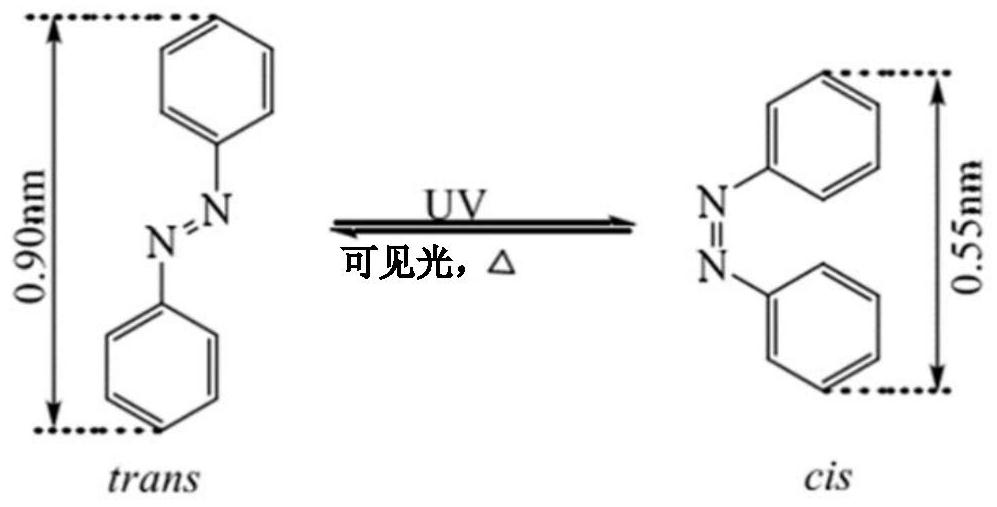
A kind of reactive azobenzene main side chain liquid crystal compound and its preparation method and application
A liquid crystal compound, azobenzene main technology, applied in the field of reactive azobenzene main and side chain liquid crystal compounds and their preparation, can solve problems such as restricting the application of azobenzene compounds, and achieve excellent crystallinity, excellent liquid crystallinity, application wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
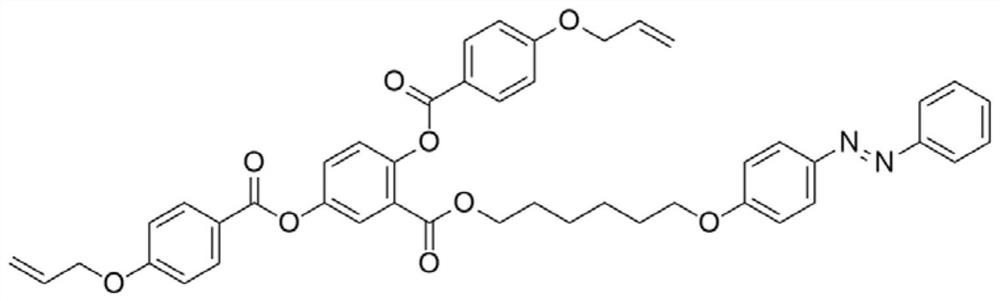
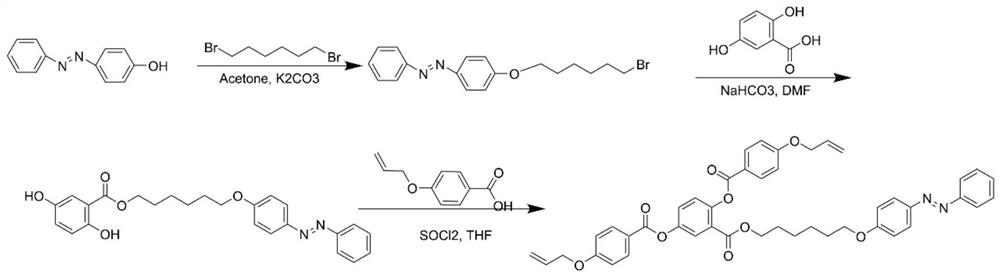
[0062] A reactive type azobenzene main side chain liquid crystal compound, prepared by the method comprising the following steps, the structural formula of the liquid crystal compound of the present invention is as follows: figure 2 As shown, the synthetic route is as image 3 Shown:
[0063] (1) Dissolve 17.52 parts by mass of 1,6-dibromohexane, 0.36 parts by mass of potassium iodide in 30 parts by volume of acetone, heat up to 60°C, add 12.45 parts by mass of anhydrous potassium carbonate, stir for 0.5h, then Under nitrogen protection, 11.89 parts by mass of 4-hydroxyazobenzene in acetone (200 parts by volume) was slowly added to the mixture through a constant pressure funnel, and stirred for 48 hours. After confirming the complete reaction of 4-hydroxybiazobenzene by TLC, the salt in the reaction mixture was removed by filtration, the filtrate was concentrated to about 50 parts by volume, and then poured into absolute ethanol to precipitate yellow crystals. The crystals ...
Embodiment 2
[0069] A reactive azobenzene main side chain liquid crystal compound is prepared by a method comprising the following steps:
[0070] (1) Dissolve 29.2 parts by mass of 1,6-dibromohexane and 0.6 parts by mass of potassium iodide in 30 parts by volume of acetone, heat up to 60°C, add 16.6 parts by mass of anhydrous potassium carbonate, stir for 0.5h, and then Under nitrogen protection, 11.89 parts by mass of 4-hydroxyazobenzene in acetone (200 parts by volume) was slowly added to the mixture through a constant pressure funnel, and stirred for 48 hours. After confirming the complete reaction of 4-hydroxybiazobenzene by TLC, the salt in the reaction mixture was removed by filtration, the filtrate was concentrated to about 50 parts by volume, and then poured into absolute ethanol to precipitate yellow crystals. The crystals were collected by filtration, washed 3 times with water (200 parts by volume), and dried in a vacuum oven at 50° C. for 24 hours to obtain 4-(((6-bromohexyl)ox...
Embodiment 3
[0076] A reactive azobenzene main side chain liquid crystal compound is prepared by a method comprising the following steps:
[0077] (1) Dissolve 17.52 parts by mass of 1,6-dibromohexane, 0.36 parts by mass of potassium iodide in 30 parts by volume of acetone, heat up to 60°C, add 12.45 parts by mass of anhydrous potassium carbonate, stir for 1.0h, then Under nitrogen protection, 11.89 parts by mass of 4-hydroxyazobenzene in acetone (200 parts by volume) was slowly added to the mixture through a constant pressure funnel, and stirred for 50 h. After confirming the complete reaction of 4-hydroxybiazobenzene by TLC, the salt in the reaction mixture was removed by filtration, the filtrate was concentrated to about 50 parts by volume, and then poured into absolute ethanol to precipitate yellow crystals. The crystals were collected by filtration, washed 3 times with water (200 parts by volume), and dried in a vacuum oven at 50° C. for 24 hours to obtain 4-(((6-bromohexyl)oxy)phenyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| breaking strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com