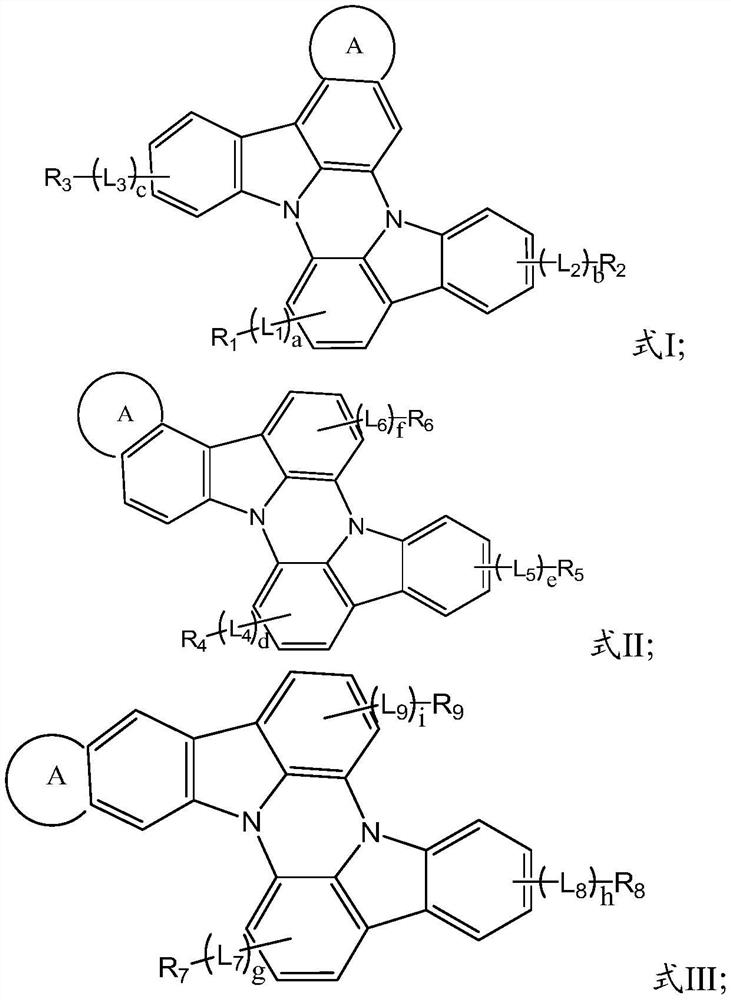
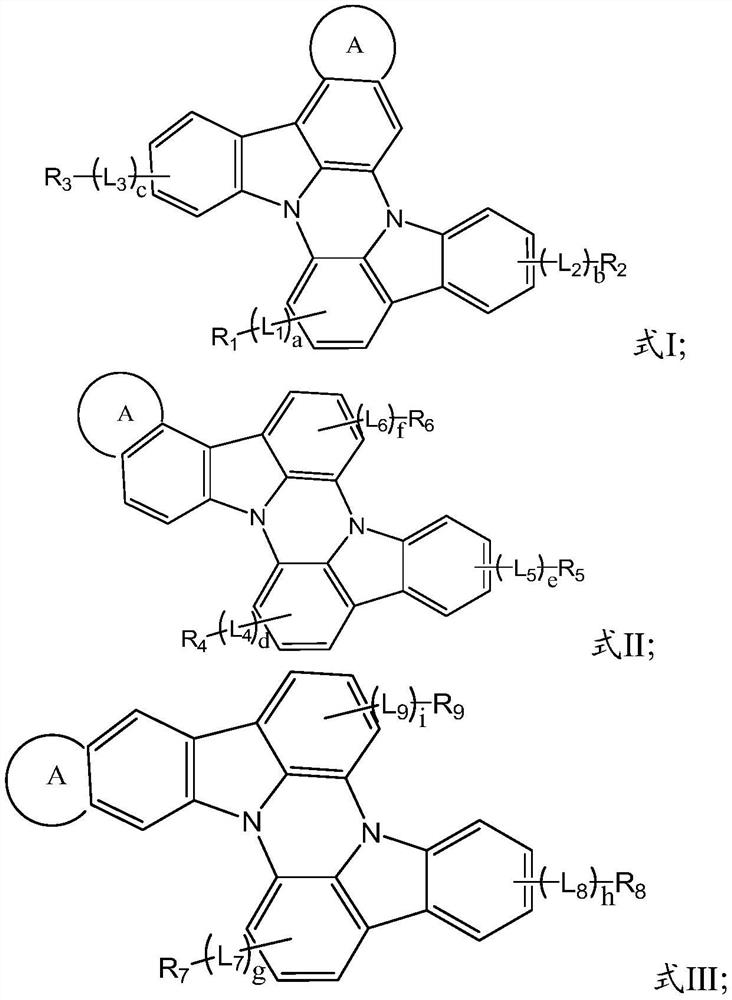
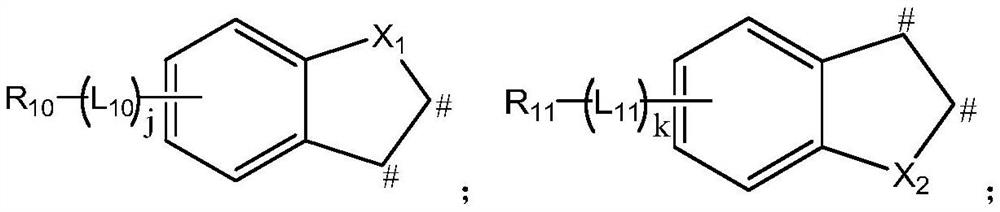A kind of n-heterobiphenyl organic compound and its application
An organic compound, biphenyl technology, applied in the field of organic electroluminescence, can solve the problem of difficult to develop doping materials, etc., to achieve improved equilibrium migration, luminous efficiency and lifetime improvement, high glass transition temperature and molecular thermal stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134] step 1)
[0135]
[0136] In a 250mL round bottom flask, compound A (15mmol), compound B (10mmol) and Pd(PPh 3 ) 4 (0.3mmol) was added to a mixture of toluene (35mL) / ethanol (25mL) and potassium carbonate (15mmol) aqueous solution (10mL), and the reaction was refluxed under nitrogen atmosphere for 12h. The resulting mixture was cooled to room temperature, added to water, and filtered through a pad of celite. The filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude The product yielded the target product P-1.
[0137] step (2)
[0138] Under argon (Ar) atmosphere, compound P-1 (20mmol), compound C (10mmol), Pd(OAc) 2 (1.6mmol), (tBu) 3 PHBF 4 (5mmol), K 2 CO 3(6mmol) into a 250mL three-necked flask, then heated in 150mL of toluene solvent and refluxed for about 6 hours. After cooling in air, water was added, the organic layer was separated and the solvent was dis...
Embodiment 2
[0142]
[0143] Compound P-1 was prepared as described above.
[0144] Under argon (Ar) atmosphere, compound P-1 (20mmol), compound E (10mmol), Pd(OAc) 2 (1.6mmol), (tBu) 3 PHBF 4 (5mmol), K 2 CO 3 (6mmol) into a 250mL three-necked flask, then heated in 150mL of toluene solvent and refluxed for about 6 hours. After cooling in air, water was added, the organic layer was separated and the solvent was distilled off. The crude product thus obtained was separated by silica gel column chromatography (using a mixed solvent of dichloromethane and hexane), and then recrystallized using a mixed solvent of toluene and hexane to obtain compound P-3 as a solid.
[0145] Characterization results of the organic compound P-3: molecular formula C 54 h 33 N 3 ;
[0146] ESI-MS(m / z)[M+1] analyzed by liquid chromatography-mass spectrometry + : The theoretical value is 724.27, the test value is 724.45; the theoretical value of elemental analysis: C 89.60, H 4.60, N 5.81; the test valu...
Embodiment 3
[0148]
[0149] In a 250mL round bottom flask, compound A (15mmol), compound D (10mmol) and Pd(PPh 3 ) 4 (0.3mmol) was added to a mixture of toluene (35mL) / ethanol (25mL) and potassium carbonate (15mmol) aqueous solution (10mL), and the reaction was refluxed under nitrogen atmosphere for 12h. The resulting mixture was cooled to room temperature, added to water, and filtered through a pad of celite. The filtrate was extracted with dichloromethane, washed with water, and dried over anhydrous magnesium sulfate. After filtration and evaporation, the crude The product yielded the target product P-4.
[0150] Under argon (Ar) atmosphere, compound P-4 (20mmol), compound E (10mmol), Pd(OAc) 2 (1.6mmol), (tBu) 3 PHBF 4 (5mmol), K 2 CO 3 (6mmol) into a 250mL three-necked flask, then heated in 150mL of toluene solvent and refluxed for about 6 hours. After cooling in air, water was added, the organic layer was separated and the solvent was distilled off. The crude product thus ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| internal quantum efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com