Compositions and methods of enhancing 5-hydroxytryptophan bioavailability
A technology of availability and biology, applied in the direction of drug combination, medical preparations containing active ingredients, drug delivery, etc., can solve problems such as broad-spectrum toxicity, physiological system impact, and impracticality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0088] method
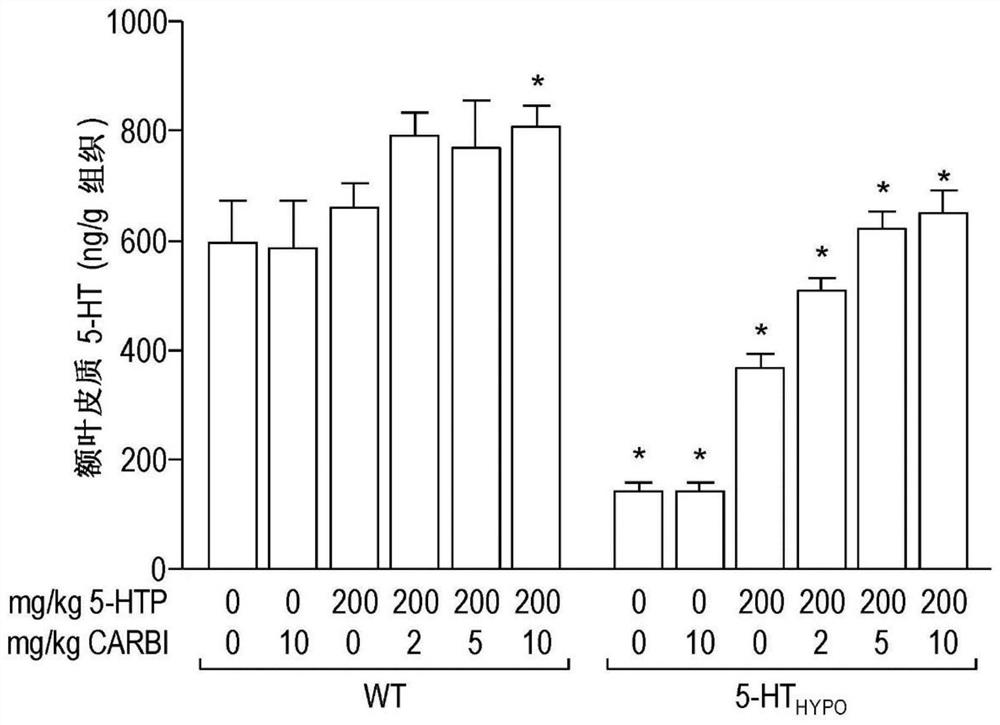
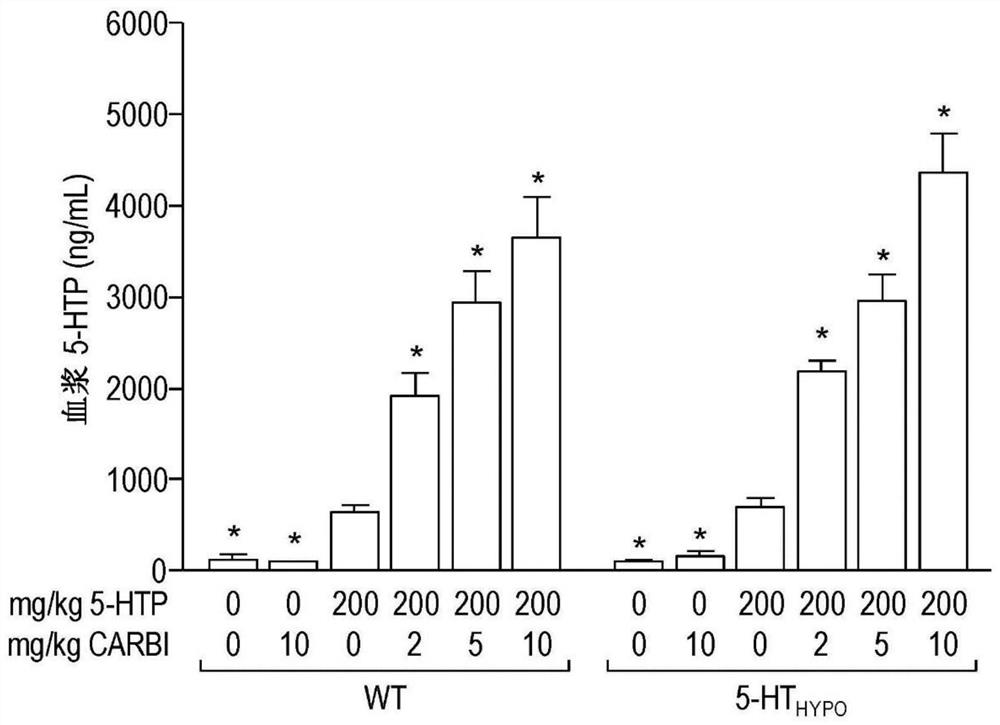
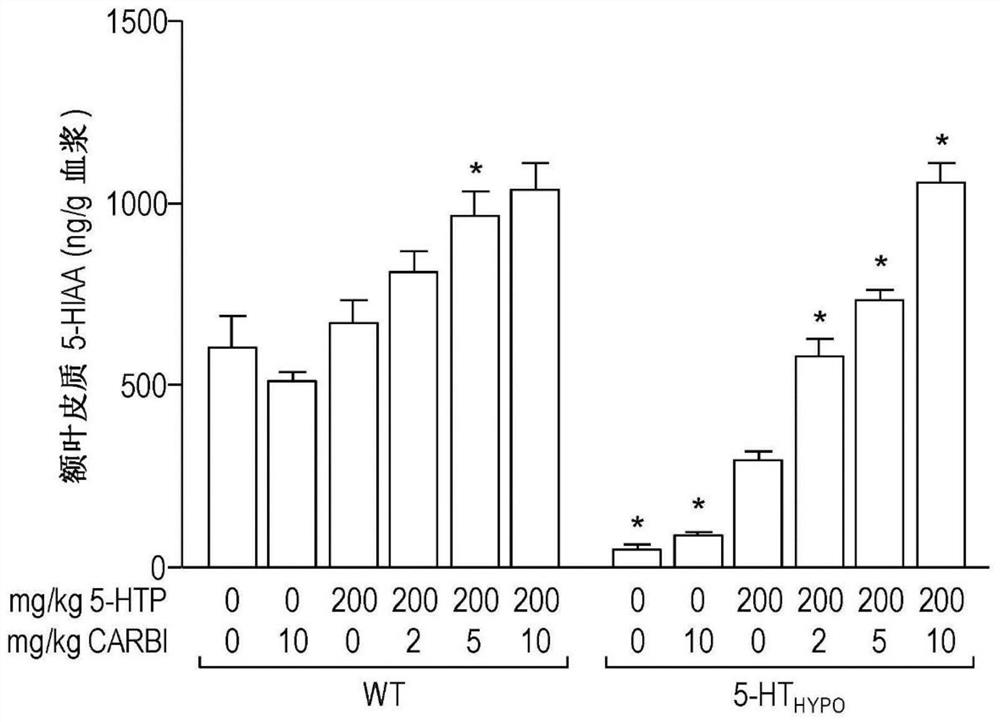
[0089] Mice: Using adult mice, wild-type (WT) mice with normal 5-HT levels and "5-HT" mice with reduced brain 5-HT synthesis and levels Hypo ” mice (Beaulieu et al., 2008). 5-HT Hypo Mice are a natural model of brain 5-HT deficiency, which is known to be a causative agent of several central nervous system disorders, such as depression and suicidality.
[0090] Drug therapy: Carbidopa is used as a PDI. To mimic the oral drug delivery that occurs in humans during treatment, 5-HTP and carbidopa were delivered via mouse chow (standard chow). This method distributes the drug over time, thereby providing a measure of "sustained release" (ie sustained release, extended release, time release, controlled release). The dose of 5-HTP was 200 mg / kg / day. In addition to 5-HTP, carbidopa was administered at doses of 2, 5, or 10 mg / kg / day to evaluate the effect of carbidopa on the outcome of 5-HTP treatment. To assess the effect of carbidopa alone, groups of mice were als...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com