Quantitative analysis method of biogenic amine neurotransmitter
A neurotransmitter, quantitative analysis technology, applied in the field of detection, can solve the problems of high price of stable isotope labels, unfavorable promotion and application, no commercialization of metabolites, etc., to avoid matrix effects, low detection cost, and high sensitivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0110] In some preferred embodiments, the preparation method of the internal reference B includes the following steps:
[0111] To an aqueous solution containing homovanillic acid, 5-hydroxyindoleacetic acid, dopa, 3-methoxydopa, vanillylmandelic acid, dihydroxyphenylacetic acid, 5-hydroxytryptophan, and vanillyllactic acid, derivatization reagent C was sequentially added and derivatization reagent B, after mixing, extract with ethyl acetate, and the extract is the internal reference B;
[0112] The derivatization reagent C comprises D3-propanol and 3-picoline, and the volume ratio of D3-propanol and 3-picoline in the derivatization reagent C is (70-80):(20-30), preferably for 77:23.
[0113] Since each substance in the internal reference B is expensive, the present invention also provides a laboratory preparation method for the internal reference B. The preparation method is simple, low in cost, and the prepared isotopic internal reference is stable.
[0114] In some prefer...
Embodiment 1
[0129] A quantitative analysis method for biological amine neurotransmitters, comprising the following steps:
[0130] 1. Take 100 μL of cerebrospinal fluid sample, add 10 μL of stable isotope marker internal reference A solution, and 50 μL of saturated sodium tetraborate aqueous solution in sequence to obtain mixed solution 1.
[0131] Among them, the internal reference A is 0.5% hydrochloric acid aqueous solution, which contains the following stable isotopes of 250nmol / L: D4-dopamine, D4-3-methoxytyramine, D6-norepinephrine, D3-epinephrine, D3-metrenephrine , D3-norepinephrine.
[0132] 2. Add derivatization reagent A and derivatization reagent B to the mixed solution 1 in sequence and mix quickly to carry out the derivatization reaction to obtain the mixed solution 2;
[0133] Wherein, derivatization reagent A is the mixed solution of n-propanol and 3-picoline (volume ratio is 77:23), and derivatization reagent B is the mixed solution of dichloromethane, propyl chloroforma...
Embodiment 2
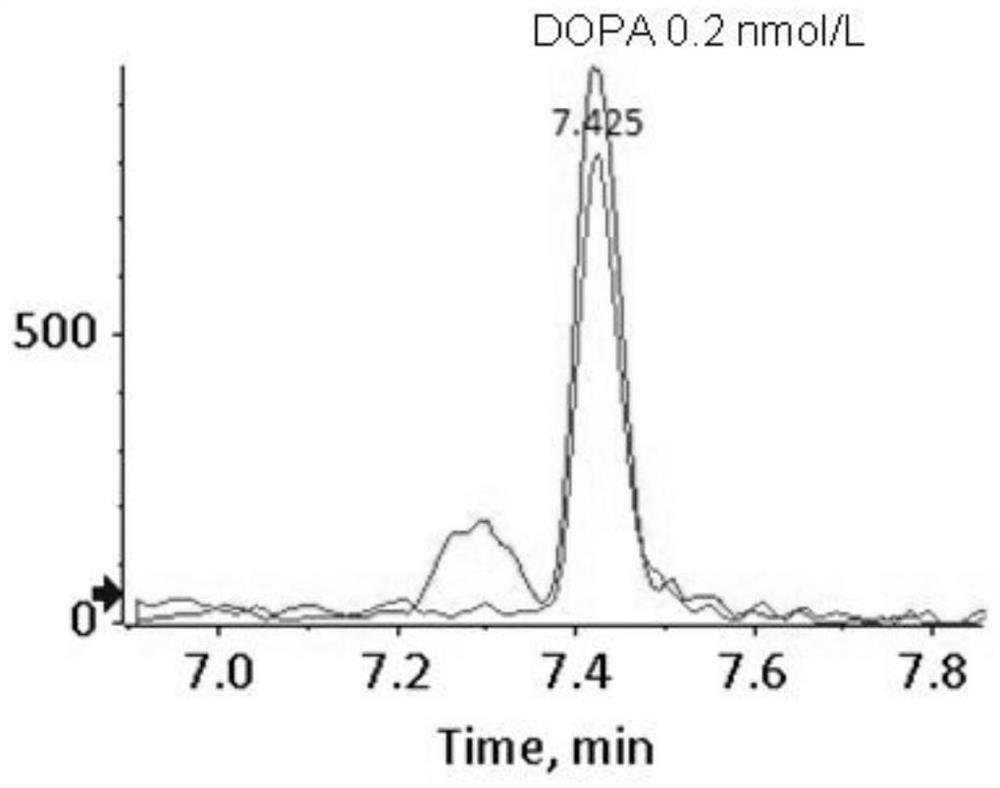
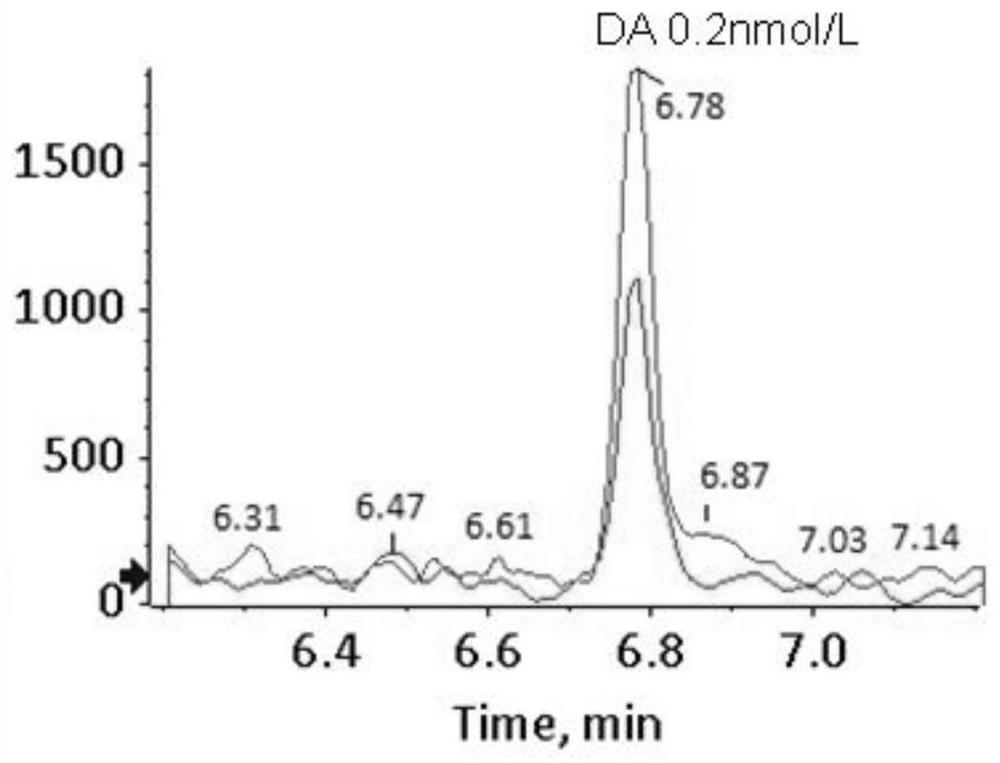
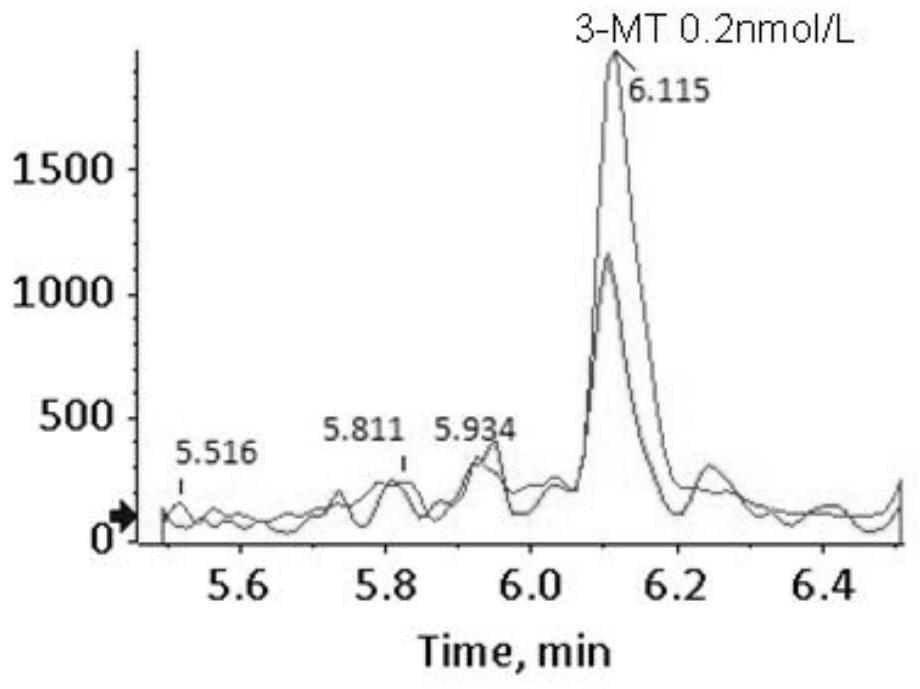
[0145] Example 2 Determination of the detection limit of 16 kinds of biological amine neurotransmitters
[0146] 1. Weigh a certain quality of 3-methoxy-4-hydroxy-phenylethylene glycol, 5-hydroxytryptamine, dopamine, 3-methoxytyramine, norepinephrine, epinephrine, metrepinephrine, normethyl Metanephrine, homovanillic acid, 5-hydroxyindoleacetic acid, dopa, 3-methoxydopa, vanillylmandelic acid, dihydroxyphenylacetic acid, 5-hydroxytryptophan and vanillyl lactic acid, each using 0.01mol / L The hydrochloric acid solution was dissolved and fixed to volume to obtain a sample solution with a concentration of 1mmol / L, and then each sample solution was diluted with 0.01mol / L hydrochloric acid solution to 0.1nmol / L, 0.2nmol / L, 0.5nmol / L, 1nmol / L, 2nmol / L, 5nmol / L to obtain sample solutions with different concentrations.
[0147] 2. Take 100 μL of the above dilutions of standard products with different concentrations, add 10 μL of 0.5% hydrochloric acid aqueous solution, and 50 μL of sa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com