EGFR (epidermal growth factor receptor) kinase inhibitor and application thereof in preparation of anti-cancer drugs
A technology of pharmacy and compound, applied in the field of novel EGFR kinase inhibitor and its application in the treatment of cancer, can solve unknown problems and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
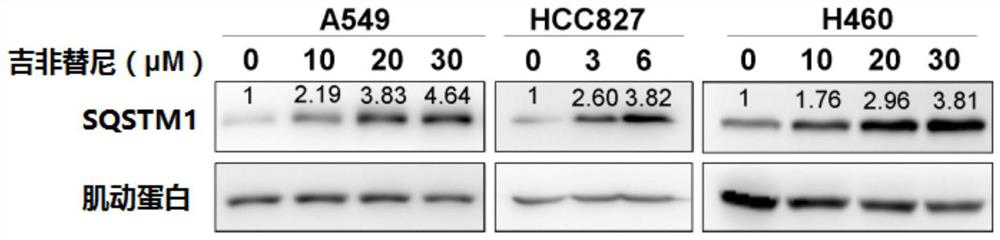
[0228] With a specified concentration (such as figure 1 Indicated) gefitinib treated non-small cell lung cancer cells (NSCLC) for 24 hours (for A549 and H460 cells) or 36 hours (for HCC827 cells), and the expression of SQSTM1 was determined by Western Blot. Actin was used as an internal reference protein. Experimental results such as figure 1 shown.
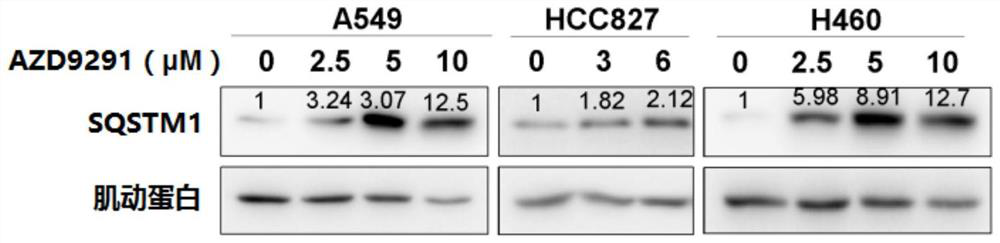
[0229] With a specified concentration (such as figure 2 Shown) AZD9291 treated A549, HCC827 and H460 cells for 24 hours, and the expression of SQSTM1 was determined by Western Blot. Actin was used as an internal reference protein. Experimental results such as figure 2 shown.
[0230] Western Blot operation steps:
[0231] Cells were lysed with a lysis buffer containing 2% SDS, 0.1% bromophenol blue, 10% glycerol, 1.5% DTT (dithiothreitol), and 0.1M tris-HCl (pH 6.8). The protein concentration in cell lysates was determined by BCA method protein quantification kit. Boil-denatured lysates were separated by SDS-PAGE, tran...
Embodiment 2
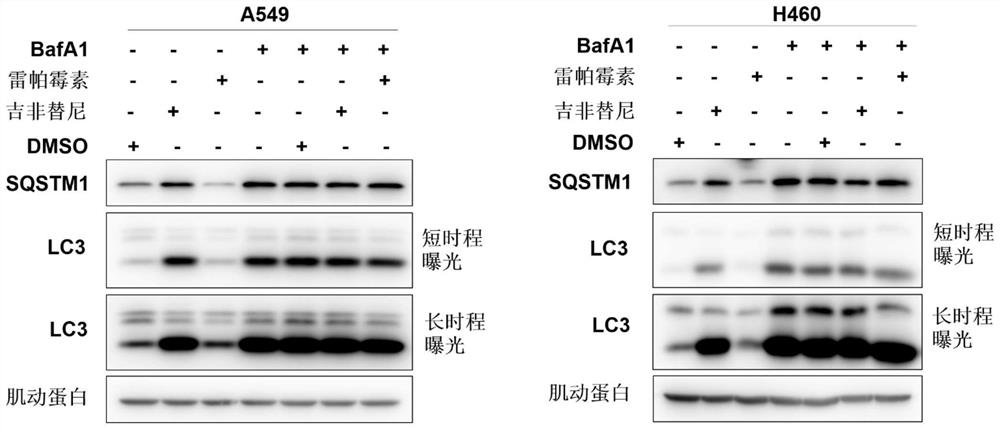
[0234] A549 and H460 cells were treated with DMSO, rapamycin, and gefitinib for 7 hours, and then BafA1 (bafilomycin A1) was administered or not to the above cells, and cultured for 5 hours. LC3 and SQSTM1 expressions were detected by Western Blot (refer to the Western Blot step in Example 1). Experimental results such as image 3 shown.
[0235] Depend on image 3 The results showed that the effect of gefitinib alone was similar to that of the autophagy inhibitor Baf A1, but opposite to that of the autophagy inducer rapamycin; after the combination of rapamycin and BafA1, the protein levels of LC3-II and SQSTM1 Both significantly increased, but the combination of gefitinib and BafA1 did not show a significant enhancement effect. The above experimental results indicated that gefitinib probably blocked the autophagic degradation of SQSTM1 by inhibiting autophagy, leading to its accumulation in cells.
Embodiment 3
[0237] A549 cells were transfected with GFP-RFP-LC3 plasmid for 48 hours, and then the cells were treated with EBSS, CQ, gefitinib or AZD9291, respectively. The spots of GFP-LC3 and RFP-LC3 were detected by immunofluorescence microscopy, and the experimental results were as follows Figure 4 As shown in A.
[0238] Quantification of LC3 yellow spots / red spots (%) is expressed as mean±SD of 3 independent experiments, n=10 cells. Statistical results such as Figure 4 Shown in B, where an asterisk (*) indicates a statistical difference (Student's t-test, P<0.05).
[0239] From the results of Examples 2 and 3, it can be seen that the inhibitory effect of gefitinib and AZD9291 on autophagy is similar to that of the known autophagy inhibitors bafilomycin A1 (Baf A1) and chloroquine (CQ), although they can upregulate LC3II expression (eg image 3 shown), but it negatively regulates the process of autophagic flux in cells (such as Figure 4 shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com