Method for catalyzing cyanosilicification reaction of ketone by using deprotonated phenyl bridged beta-ketimine lithium complex
A technology for catalyzing ketone cyanosilicone and lithium ketimine, which is applied in catalytic reactions, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problem of long time and high catalyst consumption of cyanosilication reaction , Catalyst preparation complex and other issues, to achieve the effect of efficient reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment one: [L ph’ Li 4 (THF) 4 ] 2 Catalytic Reduction of Acetophenone and TMSCN
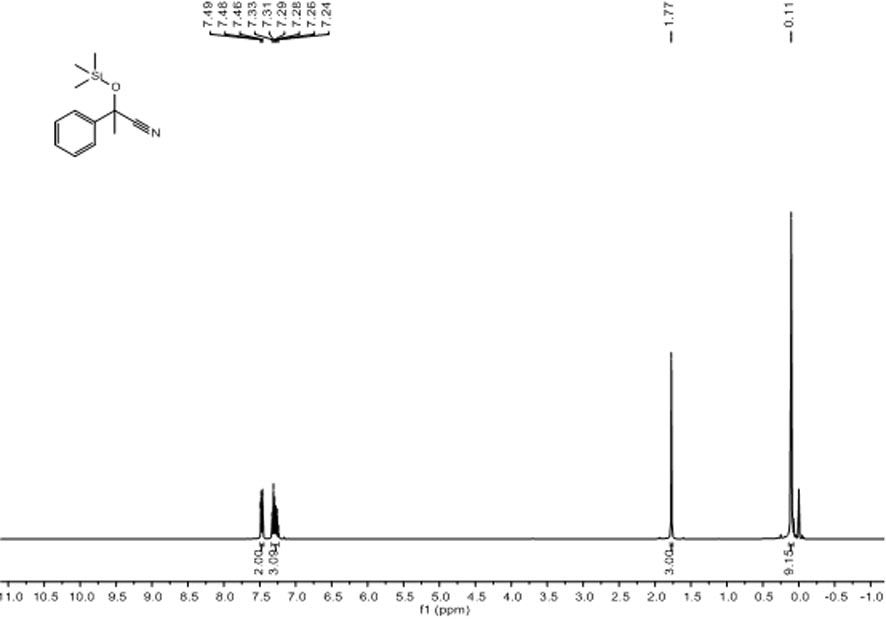
[0027] Under nitrogen atmosphere, add catalyst 0.6 mg (0.0005 mmol, 0.05%) to the reaction flask after dehydration and deoxygenation treatment, add acetophenone (116.6 μL, 1.0 mmol), TMSCN (137.6 μL, 1.1 mmol), after reacting at room temperature for 15 min, draw a drop into the NMR tube with a dropper, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 99%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) δ 7.49-7.46 (m, 2H, Ar H ),7.33-7.24 (m, 3H, Ar H ), 1.77 (s, 3H, C Me ), 0.11 (s, 9H, Si(C H 3 ) 3 ), the spectrum see figure 1 .
Embodiment 2
[0028] Embodiment two: [L ph’ Li 4 (THF) 4 ] 2 Catalytic Reduction of p-Methylacetophenone and TMSCN
[0029] Under a nitrogen atmosphere, add 0.6 mg (0.0005 mmol) of the catalyst to the reaction flask after dehydration and deoxygenation, and then add p-methylacetophenone (133.5 μL, 1.0 mmol), TMSCN (137.6 μL, 1.1 mmol), after reacting at room temperature for 15 min, draw a drop into the NMR tube with a dropper, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 99%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) δ 7.46 (d, 3 J H-H = 8.3 Hz, 2H, Ar H ),7.22 (d, 3 J H-H = 8.0 Hz, 2H, Ar H ), 2.38 (s, 3H, Ar-C H 3 ), 1.86 (s, 3H, C Me ),0.19 (s, 9H, Si(C H 3 ) 3 ).
Embodiment 3
[0030] Embodiment three: [L ph’ Li 4 (THF) 4 ] 2 Catalyzed reduction reaction of o-fluoroacetophenone and TMSCN
[0031] Under a nitrogen atmosphere, add 0.6 mg (0.0005 mmol) of the catalyst to the reaction flask after dehydration and deoxygenation, and then add o-fluoroacetophenone (124.1 μL, 1.0 mmol), TMSCN (137.6 μL, 1.1 mmol) with a pipette gun ), after reacting at room temperature for 15 min, draw a drop into the NMR tube with a dropper, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 99%. NMR data of the product: 1 H NMR (400 MHz, CDCl 3 ) δ 7.54-7.50 (m, 1H, Ar H ), 7.30-7.25(m, 1H, Ar H ), 7.13-7.09 (m, 1H, Ar H ), 7.04-6.99 (m, 1H, Ar H ), 1.87 (s, 3H,C Me ), 0.19 (s, 9H, Si(C H 3 ) 3 ).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap