New 2' and/or 5' amino-acid ester phosphoramidate 3'-deoxy adenosine derivatives as Anti-cancer compounds
一种化合物、烷基的技术,应用在作为抗癌化合物的新的2’和/或5’-氨基酸酯氨基磷酸酯3’-脱氧腺苷衍生物领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0356] Preparation of 3'-deoxy-2-fluoroadenosine:
[0357] H 2 O / CH 3 A solution of CN (1:9; 1.4 mL) and then α-AIBBr (4.10 mL, 28.05 mmol) was added to dry 2-fluoroadenosine (2.0 g, 7.01 mmol) in anhydrous CH 3 CN (50 mL) and continued stirring at room temperature (20 °C). After 1 h, carefully add saturated NaHCO 3 solution, and the solution was extracted with EtOAc (2x100 mL). The combined organic phases were washed with brine (1x50 mL). The aqueous phase was extracted with EtOAc (2x50 mL), and the 2 SO 4 The combined organic phases were dried on NaCl, filtered and evaporated to give a white gum. The crude mixture was dissolved in THF / H 2 O (4 / 1, 50mL) mixture, and with 60mL of Amberlite (2x OH - ) resin (previously washed well with THF) was stirred for 1 hour. The solution was then filtered and the resin was carefully washed with THF. The combined filtrates were evaporated and the residue was crystallized from EtOH to afford 2',3'-dehydro-2-fluoroadenosine (1.13 ...
Embodiment 2
[0540] Example 2 - Cytotoxicity
[0541] The anticancer efficacy of exemplary compounds embodying the invention was assessed as follows.
[0542] Using the CellTiterGlo (CTG, Promega-G7573) assay, an in vitro viability assay was performed over 72 hours to assess the effect of compounds on cell viability in 7 selected cell lines. Assays were performed in duplicate, with 3.16-fold titration in 96-well plates, treating compounds at 9 points over approximately 72 hours. The starting compound concentration was 198 mM. Cell viability assays using CellTiterGlo in 96-well plates were performed. Compound treatments were performed in duplicate for 72 hours under standard growth conditions. Compounds were dissolved to 40 mM and thawed 100%. Compounds were serially diluted 3.16-fold in thawed DMSO and warmed to 37°C before dissolving in medium (2 μl + 200 μl). After dissolving the compounds in the medium (the medium was also warmed to 37°C). In duplicate, the medium containing the c...
Embodiment 3
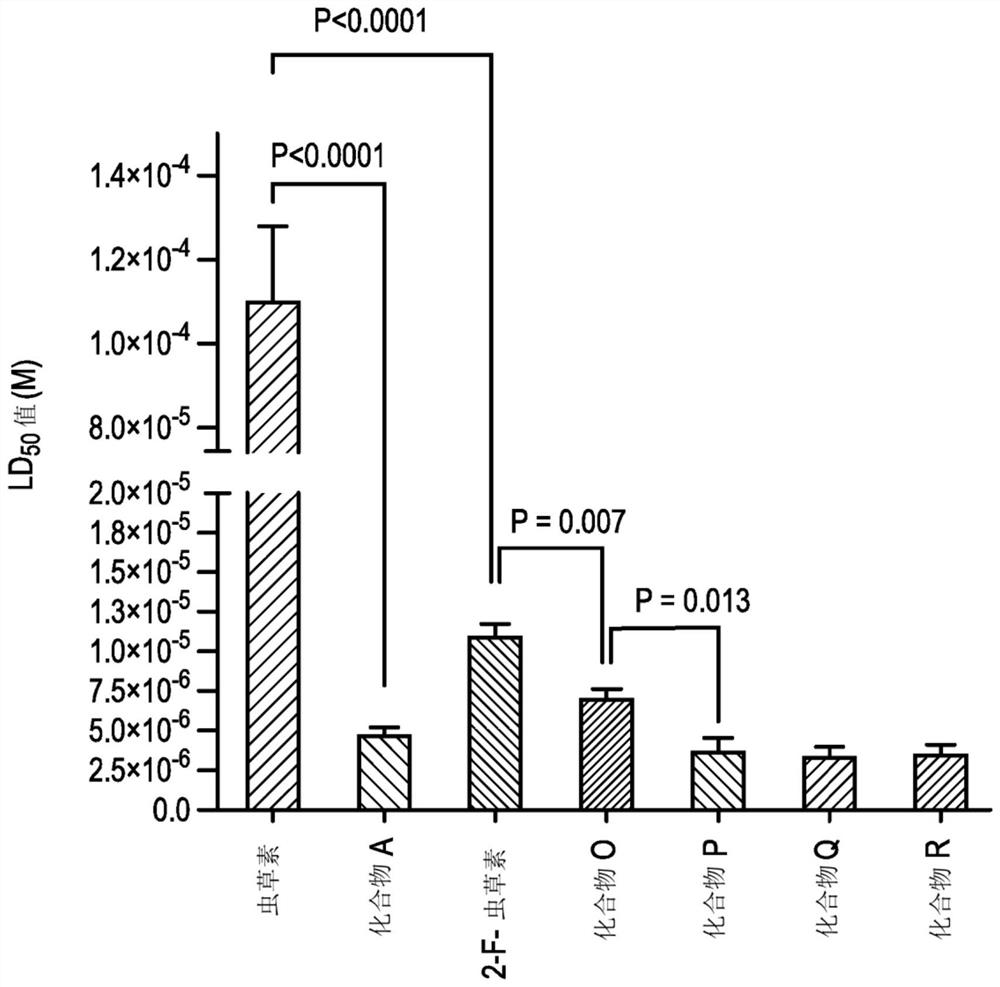
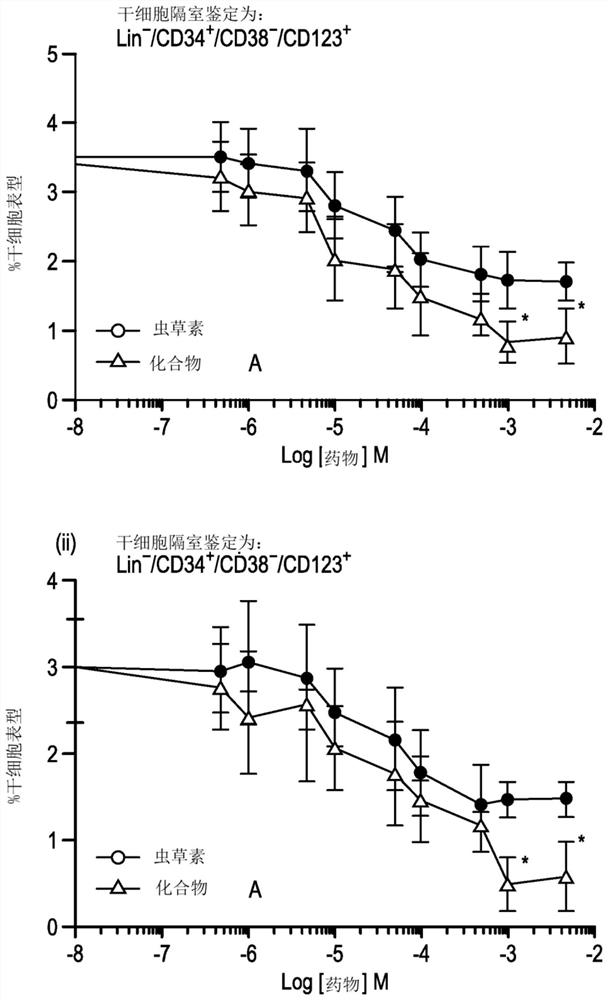
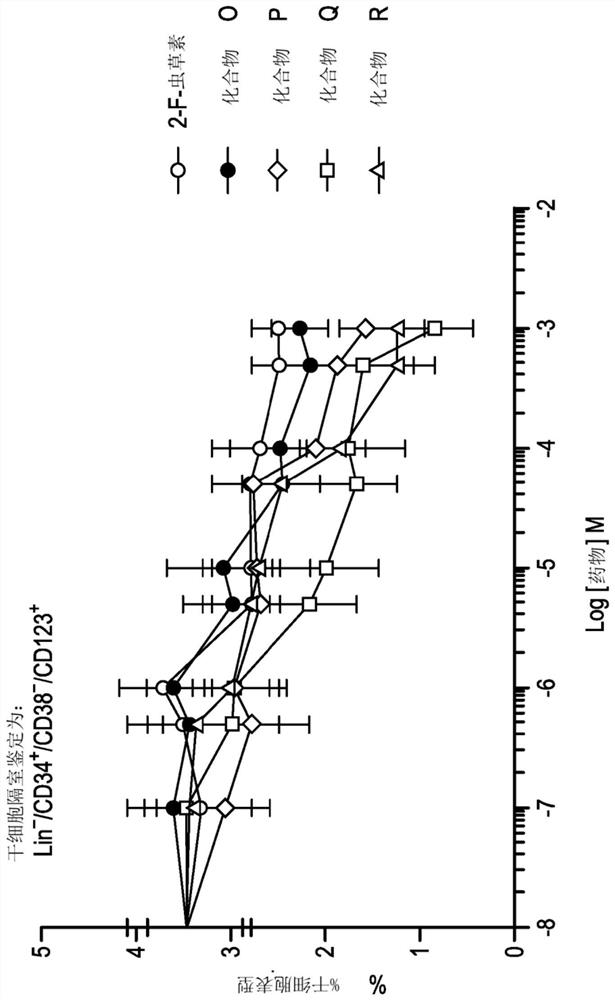
[0587] Example 3 - Evaluation of Cytotoxicity and Cancer Stem Cell Activity
[0588] A further comparative analysis of the toxicity of the compound in the acute myeloid leukemia (AML) cell line KG1a over an expanded dose range was performed and the effect of the compound on the leukemia stem cell (LSC) compartment within the KG1a cell line was assessed across the dose range relative impact.
[0589] Materials and methods
[0590] KG1a cell culture conditions
[0591] The KG1a cell line was maintained in RPMI medium (Invitrogen, Paisley, UK) supplemented with 100 units / ml penicillin, 100 μg / ml streptomycin and 20% fetal bovine serum. Subsequently, the cells were aliquoted (10 5 cells / 100 μl) into a 96-well plate, and in the presence of nucleoside analogs and their respective proTides having concentrations experimentally determined for each series of compounds, at 37 °C for 72 hours in a humidified 5% carbon dioxide atmosphere. In addition, a control culture in which no d...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap