A kind of conjugated seven-membered fluoroboron dipyrrole fluorescent dye and its synthesis method
A technology of elemental fluoroboron dipyrrole and fluorescent dye, applied in the field of conjugated seven-membered fluoroboron dipyrrole fluorescent dye and its synthesis, can solve problems restricting the application of cell imaging, and achieve easy control of reaction conditions, mild reaction conditions, and universal The effect of applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
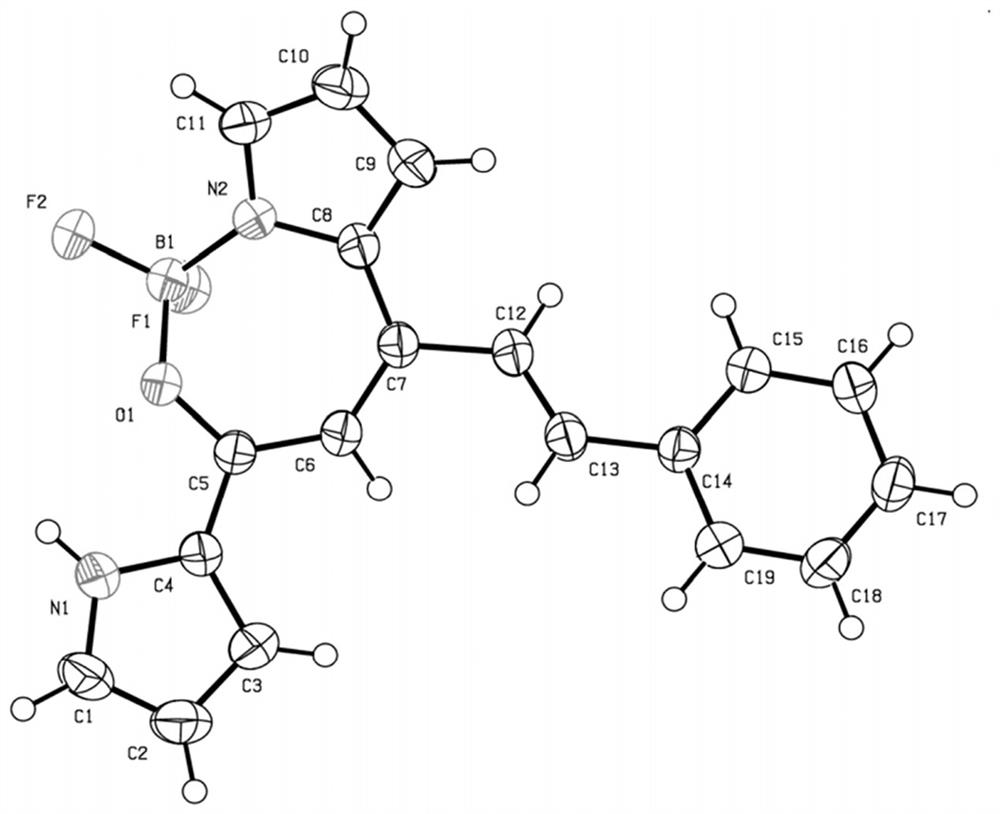
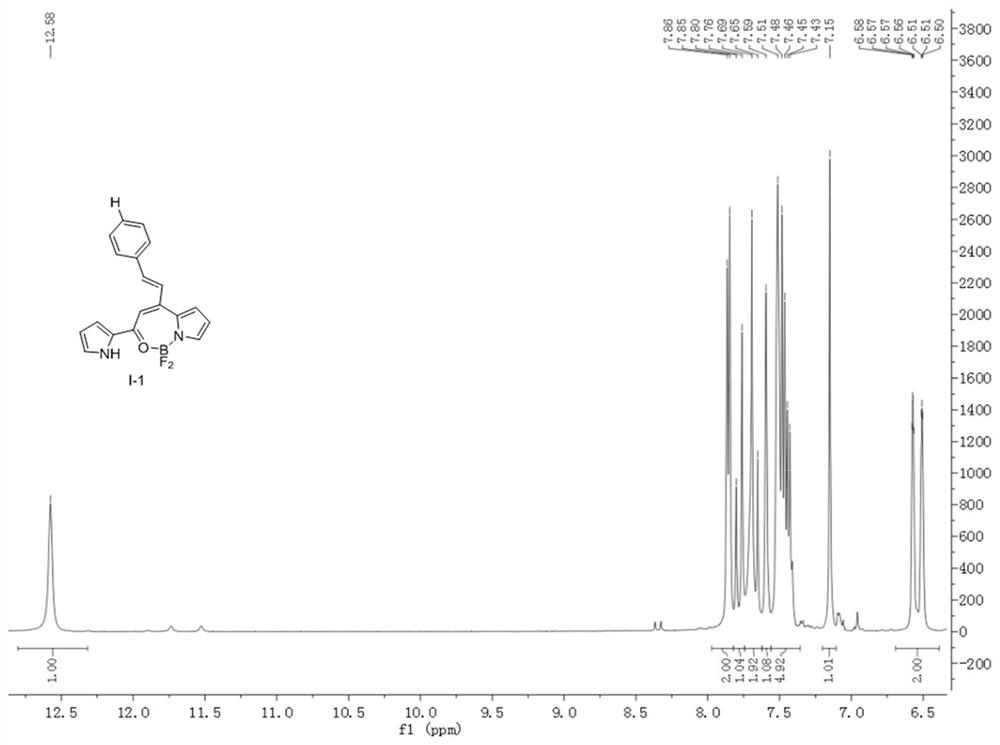
[0037] Weigh compound 1 seven-membered fluoroboron dipyrrole compound (248mg, 1mmol), take 30.00ml toluene, mix and dissolve, then add benzaldehyde (0.1ml, 1mmol), piperidine (0.09ml, 1mmol), acetic acid (0.06ml , 1 mmol), heated and stirred at 80°C for 2 hours to complete the reaction, the reactant was rotary evaporated, and purified by column chromatography to obtain a dark purple solid I-1 (111 mg), with a yield of 33.0%.
[0038]
Embodiment 2
[0040] Weigh compound 1 seven-membered fluoroboron dipyrrole compound (248mg, 1mmol), take 30.00ml toluene, mix and dissolve, then add benzaldehyde (0.2ml, 2mmol), piperidine (0.09ml, 1mmol), acetic acid (0.06ml , 1 mmol), heated and stirred at 80°C for 2 hours to complete the reaction, the reactant was rotary evaporated, and purified by column chromatography to obtain a dark purple solid I-1 (125 mg), with a yield of 37.2%. When the amount of benzaldehyde increased by 2 times relative to Example 1, the yield increased by 4.2%. X single crystal diffractogram and hydrogen spectrogram are the same as embodiment 1.
[0041]
Embodiment 3
[0043] Weigh compound 1 seven-membered fluoroboron dipyrrole compound (248mg, 1mmol), take 30.00ml toluene, mix and dissolve, then add benzaldehyde (0.2ml, 2mmol), piperidine (0.18ml, 2mmol), acetic acid (0.12ml , 2mmol), heated and stirred at 80°C for 2 hours to complete the reaction, the reactant was rotary evaporated, and purified by column chromatography to obtain a deep purple solid I-1 (126mg), with a yield of 37.5%. When the amounts of piperidine and acetic acid were increased by 2 times compared to Example 2, the yield had no significant change. X single crystal diffractogram and hydrogen spectrogram are the same as embodiment 1.
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com