Preparation method of catalyst for preparing methanol by low-temperature plasma-optical coupling methane and method for preparing methanol
A low-temperature plasma and catalyst technology, which is used in oxidation reaction preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as low yield and harsh conditions, and achieve the effects of increasing yield, inhibiting decomposition, and being easy to activate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0025] Example 1
[0026] Put Cu(NO 3 ) 2 ·3H 2 10 g of O and 5 g of BTC (trimellitic acid) were dissolved in a mixed solution of deionized water (83.3 mL), ethanol (83.3 mL) and DMF (83.3 mL), and magnetically stirred at room temperature for 30 min. The uniformly mixed solution was transferred to a stainless steel autoclave lined with polytetrafluoroethylene and crystallized at 80°C for 24 hours. The product was filtered and washed, and soaked in DMF for 3 days. DMF was changed every 12 hours to remove residual reagents. The samples were soaked in acetone for 3 days at room temperature, and the acetone was changed every 12 hours. After filtering the solid, it was dried overnight at 80° C. to obtain a Cu-C precursor. Then the Cu-C precursor was pyrolyzed in flowing nitrogen at 400° C. for 3 h, and naturally cooled to obtain a Cu-C catalyst, which was denoted as Cu-C-400.
Example Embodiment
[0027] Example 2
[0028] Put Cu(NO 3 ) 2 ·3H 2 10 g of O and 5 g of BTC (trimellitic acid) were dissolved in a mixed solution of deionized water (83.3 mL), ethanol (83.3 mL) and DMF (83.3 mL), and magnetically stirred at room temperature for 30 min. The uniformly mixed solution was transferred to a stainless steel autoclave lined with polytetrafluoroethylene and crystallized at 80°C for 24 hours. The product was filtered and washed, and soaked in DMF for 3 days to remove residual reagents. The samples were soaked in acetone for 3 days at room temperature and replaced every 12 hours. After filtering the solid, it was dried overnight at 80° C. to obtain a Cu-C precursor. Then, the Cu-C precursor was pyrolyzed in flowing nitrogen at 500° C. for 3 h, and cooled naturally to obtain a Cu-C catalyst, which was denoted as Cu-C-500.
Example Embodiment
[0029] Example 3
[0030] Put Cu(NO 3 ) 2 ·3H 2 10 g of O and 5 g of BTC (trimellitic acid) were dissolved in a mixed solution of deionized water (83.3 mL), ethanol (83.3 mL) and DMF (83.3 mL), and magnetically stirred at room temperature for 30 min. The uniformly mixed solution was transferred to a stainless steel autoclave lined with polytetrafluoroethylene and crystallized at 80°C for 24 hours. The product was filtered and washed, and soaked in DMF for 3 days to remove residual reagents. The samples were soaked in acetone for 3 days at room temperature and replaced every 12 hours. After filtering the solid, it was dried overnight at 80° C. to obtain a Cu-C precursor. Then the Cu-C precursor was pyrolyzed in flowing nitrogen at 600° C. for 3 h, and naturally cooled to obtain a Cu-C catalyst, which was denoted as Cu-C-600.
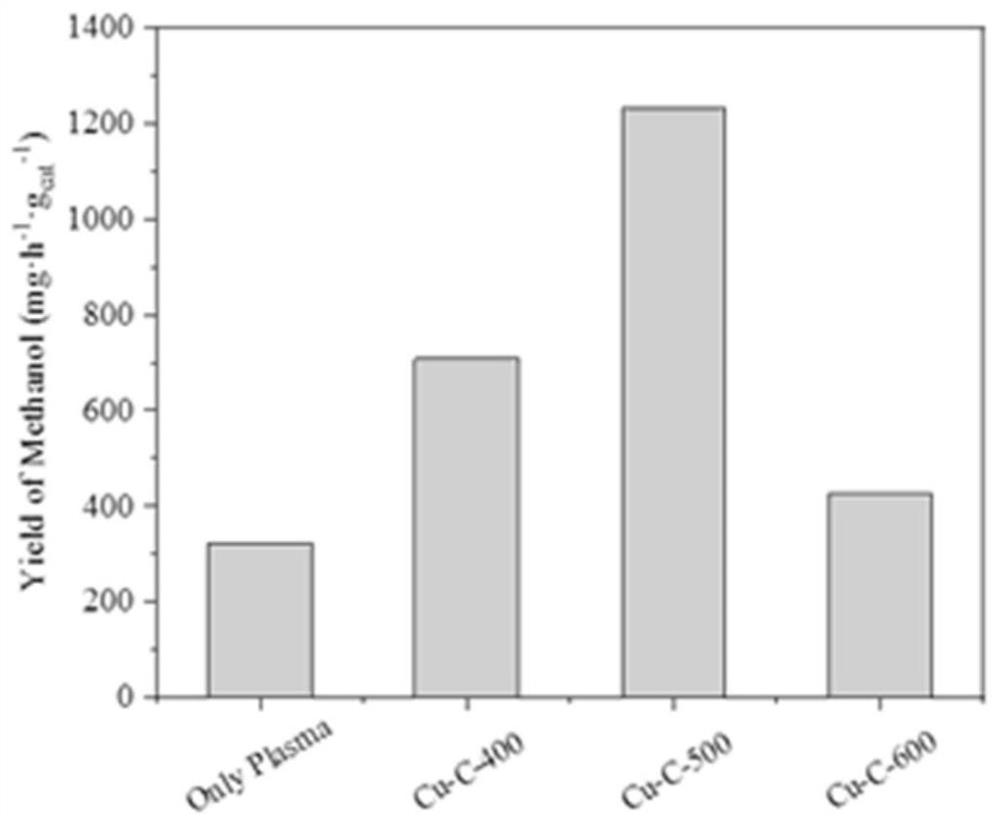
[0031] The following is an example of low-temperature plasma-optical coupling of methane to methanol.
[0032] The catalysts prepared in Examples 1-3 were e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com