Application of amomum maximum roxb extract in preparation of alpha-glucosidase inhibitor drugs
A technology of glucosidase and cardamom, applied in the field of medicinal chemistry, to achieve the effects of reducing production and absorption, protecting pancreatic function, and slowing blood sugar rise
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
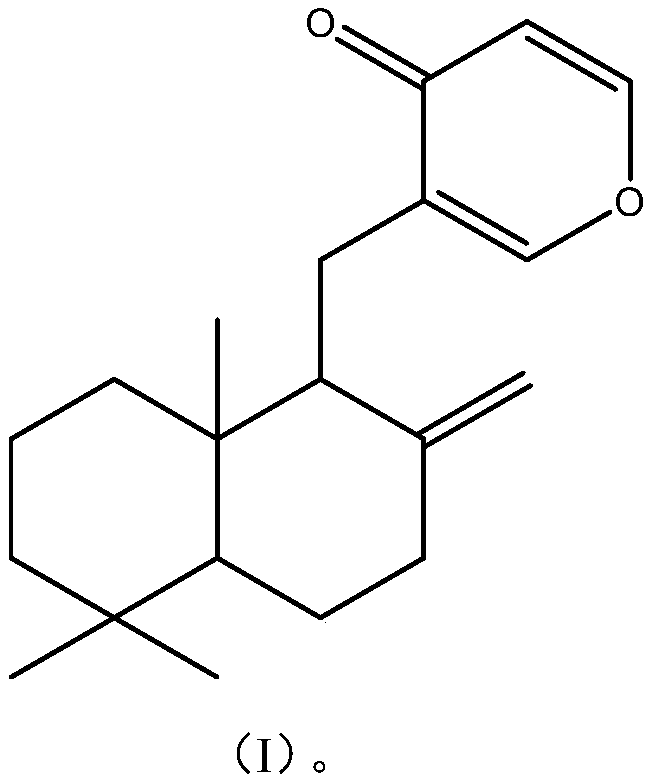
[0032] The preparation of embodiment 1 formula I compound
[0033] Take 2 kg of dry cardamom rhizomes and crush them, heat and reflux them with 5L of 95% ethanol for three times, combine the three extracts and concentrate under reduced pressure until there is no alcohol smell, add pure water until the total volume is 1L, and use an equal volume of ethyl acetate The ester was extracted three times, and the ethyl acetate extracts were combined and concentrated to dryness under reduced pressure.
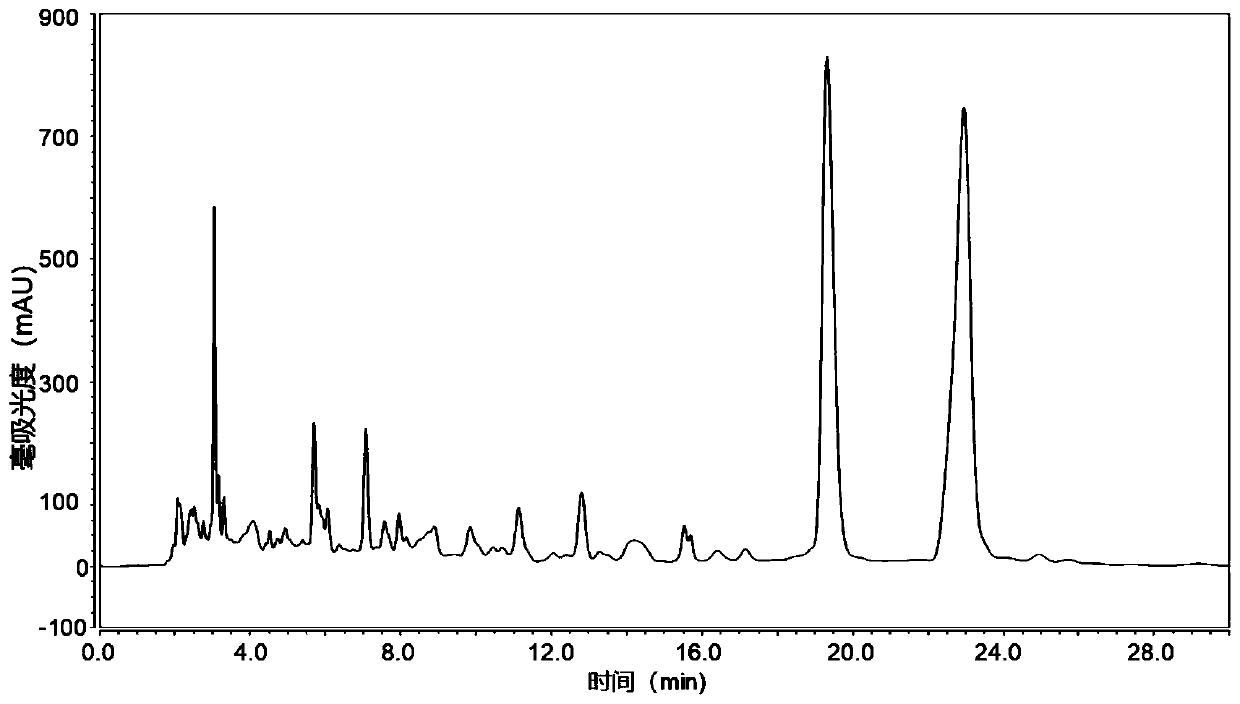
[0034] The ethyl acetate extract was subjected to silica gel column chromatography with petroleum ether-acetone gradient elution. Collect petroleum ether-acetone 9:1 eluted fractions, and recover to dryness under reduced pressure. Then through silica gel column chromatography, dichloromethane-methanol gradient elution, collect 98:2 elution fraction, this fraction utilizes preparative liquid chromatography to carry out separation, as figure 1 Shown is the liquid chromatogram of the fra...
Embodiment 2
[0035] The preparation of embodiment 2 formula I compound
[0036]Take 2 kilograms of dried cardamom rhizomes and grind them, extract by percolation with 20L 95% ethanol, combine the alcohol extracts, concentrate under reduced pressure to about 2L, add 500 grams of pretreated macroporous resin (resin model can be D101, AB -8, HPD-100 and HP20, etc.). After fully volatilizing to remove ethanol, pack the column, ethanol-water gradient elution (0, 30%, 60%, 80% and 95% ethanol), the fraction eluted with 80% ethanol is the target fraction, concentrated to dryness under reduced pressure; Column chromatography, dichloromethane-methanol gradient elution, collect 98:2 elution fraction, the fraction is separated by preparative liquid chromatography, the mobile phase is methanol-water 85:15, the flow rate is 6ml / min, the chromatographic column is RP-C18 chromatographic column (10*250mm), the detection wavelength is set at 280nm, and the chromatographic peaks with a retention time of 18...
Embodiment 3
[0037] Embodiment 3 formula I compound structure identification
[0038] The structure of the compound of formula I was determined by nuclear magnetic resonance spectrum and mass spectrometry analysis.
[0039] Its carbon spectrum and hydrogen spectrum data are as follows:
[0040] 1 H-NMR (600MHz, CDCl3) δ7.67(d, J=6.0Hz, 1H), 7.56(s, 1H), 6.33(d, J=6.0Hz, 1H), 4.82(s, 1H), 4.64( s,1H),2.69(d,J=16.2Hz,1H),2.45(dd,J=11.4,16.2Hz,1H),2.40-2.36(m,1H),2.04-1.95(m,2H),1.89 -1.85(m,1H),1.78-1.73(m,1H),1.64-1.56(tq,J=3.0,13.8Hz,1H),1.55-1.48(m,1H),1.43-1.38(m,1H) ,1.37-1.32(dq,J=4.2,12.6Hz,1H),1.24-1.15(m,3H),0.88(s,3H),0.82(s,3H),0.78(s,3H).
[0041] 13 C-NMR (150MHz, CDCl3) δ178.70(-CO-,C-13), 154.61(=CH-,C-15), 152.99(=CH-,C-16), 147.70(C,C-8 ),129.85(C,C-12),116.36(=CH-,C-14),107.77(=CH-,C-17),55.54(CH,C-5),53.96(CH,C-9) ,42.06(CH2,C-3),39.98(C,C-10),39.01(CH2,C-1),38.07(CH2,C-7),33.63(CH3,C-18),33.63(C, C-4), 24.37(CH2,C-6), 21.72(CH3,C-19), 19.54(CH2,C-11), 19.36(CH2,C-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com