Kit for joint detection of SARS-CoV-2 antigen and antibody and preparation method of kit
A combined detection, antigen-antibody technology, applied in the field of immunoanalysis medicine, can solve the problems of not being able to meet the needs of screening patients with early fever, long detection window period, and low sensitivity, and achieve easy judgment of results, high sensitivity, and short window period Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 kit of the present invention
[0035] In this embodiment, ELISA is used for detection, and the specific operations are as follows:
[0036] Step 1: Prepare high-affinity N antibody
[0037] Obtain high-purity N antigen:
[0038] (1) Clone the gene sequence of the novel coronavirus COVID-19NP protein, connect it into the expression vector pET-30a, construct the recombinant expression vector pET-30a-NP (with a 6×His tag at the N-terminal), and transform the recombinant expression plasmid into BL21 For healthy cells, pick a single colony and place it in 3ml of LB liquid medium containing ampicillin sodium, shake it overnight at 37°C, inoculate it in 250ml of fresh LB liquid medium the next day, and culture it at 37°C and 160r / min for 4h to logarithmic During the growth period, add 150 μl of 1M IPTG induction solution, and induce for 13 hours at 15°C.
[0039] (2) Collect the induced cells by centrifuging at 6000 r / min for 10 min at 4°C; r...
Embodiment 2
[0081] Embodiment 2 The using method of kit of the present invention
[0082] Step 1: Adding samples: Take the N antibody of SARS-CoV-2 virus and the S antigen microplate of SARS-CoV-2 virus. 2 wells of the N antibody positive control and 2 wells of the SARS-CoV-2 virus S antigen positive control, add 50ul to each well, add 50ul of the sample to each well of the sample well, then add 50ul of the sample diluent, mix well, and affix a seal Plate membrane, incubate at 37°C for 60 minutes;
[0083] Step 2: Wash the plate, shake off the reaction solution, wash the plate 5 times with the diluted washing solution, and finally dry it on clean absorbent paper;
[0084] Step 3: Add enzyme, except for blank wells, add horseradish peroxidase-labeled SARS-CoV-2 virus S antigen or SARS-CoV-2 virus N antibody to each well, affix the sealing film, and statically Place at 37°C for 30 minutes.
[0085] Step 4: The method is the same as step 2.
[0086] Step 5: Before use, mix TMB Chromogeni...
Embodiment 3
[0090] The clinical experiment contrast of embodiment 3 kits of the present invention
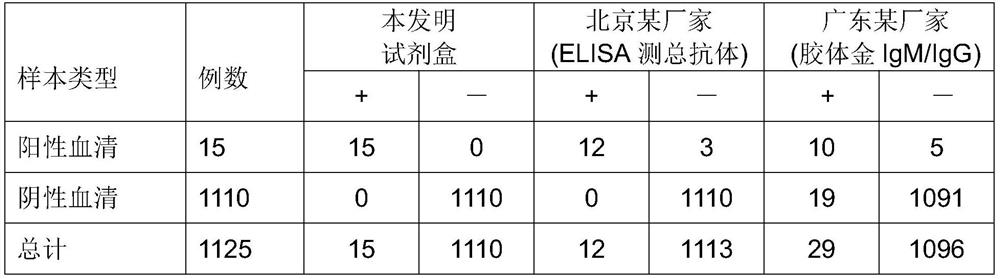
[0091] 1. The clinical experiment comparison of the kit of the present invention, the experimental results are shown in the following table:
[0092] Table 2 kit of the present invention detects serum sample and colloidal gold measurement result comparison
[0093]
[0094] Table 3 Kit of the present invention and ELISA and colloidal gold sensitivity and specificity evaluation
[0095] This kit ELISA colloidal gold sensitivity 100% 80.0% 66.7% specificity 100% 100% 98.3%
[0096] The results show that the sensitivity and specificity of the kit of the present invention are higher than those of ELISA total antibody and colloidal gold method for detecting IgM and IgG antibody detection reagents.
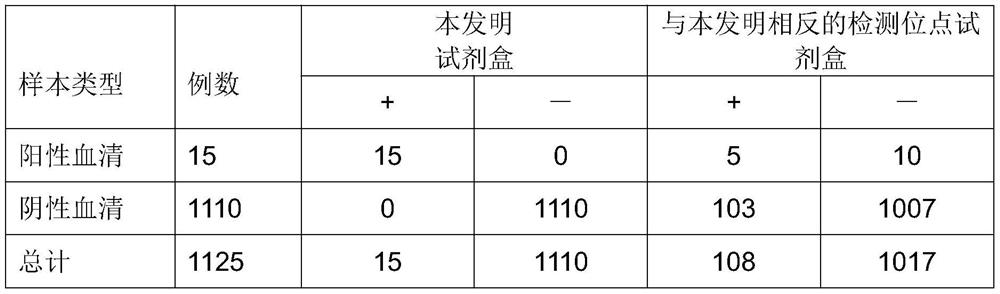
[0097] 2. Sensitivity evaluation of different kits for samples with different disease courses
[0098] In order to evaluate the sensitivity of each detection...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com